ЬтФПФкШн
ЁОЬтФПЁПЛЏбЇЗДгІдРэжЊЪЖдкжИЕМЙЄвЕЩњВњКЭЛЗБЃЕШЗНУцОпгаживЊзїгУЃЌЧыЛиД№ЯТСаЮЪЬтЃК
ЃЈ1ЃЉвбжЊШШЛЏбЇЗНГЬЪНЃК
Ђй2C(s)+H2(g) = C2H2(g) ЁїH1
ЂкC(s)+O2(g) = CO2(g) ЁїH2
ЂлH2(g) +![]() O2(g) = H2O(l) ЁїH3
O2(g) = H2O(l) ЁїH3
дђБэЪОC2H2ШМЩеШШЕФШШЛЏбЇЗНГЬЪНЮЊ____________________________ЁЃ
ЃЈ2ЃЉРћгУВтбЙЗЈдкИеадЗДгІЦїжаМгШы2molSO2ЁЂ1molO2ЃЌбаОПTЁцЪБЃЌЗДгІ2SO2 (g) +O2(g) ![]() 2 SO3(g) ЁїH<0ЃЌЬхЯЕЕФзмбЙЧПpЫцЪБМфtЕФБфЛЏШчЯТБэЫљЪОЃК
2 SO3(g) ЁїH<0ЃЌЬхЯЕЕФзмбЙЧПpЫцЪБМфtЕФБфЛЏШчЯТБэЫљЪОЃК
ЗДгІЪБМф/min | 0 | 5 | 10 | 15 | 20 | 25 | 40 |
бЙЧП/kPa | 20.0 | 19.5 | 18.3 | 16.0 | 16.0 | 16.0 | 22.0 |
ЂйЦНКтЪБЃЌSO2ЕФзЊЛЏТЪІС= _________ %ЃЛ
ЂкTЁцЪБЃЌЗДгІ2SO2 (g) +O2(g) ![]() 2 SO3(g)ЕФЦНКтГЃЪ§Kp= ________kPaЃ1[ЦјЬхЗжбЙЃЈpЗжЃЉ=ЦјЬхзмбЙЃЈpзмЃЉЁСЬхЛ§ЗжЪ§ЃЌKpЮЊвдЗжбЙБэЪОЕФЦНКтГЃЪ§ЃЌМЦЫуНсЙћБЃСє1ЮЛаЁЪ§]ЁЃ
2 SO3(g)ЕФЦНКтГЃЪ§Kp= ________kPaЃ1[ЦјЬхЗжбЙЃЈpЗжЃЉ=ЦјЬхзмбЙЃЈpзмЃЉЁСЬхЛ§ЗжЪ§ЃЌKpЮЊвдЗжбЙБэЪОЕФЦНКтГЃЪ§ЃЌМЦЫуНсЙћБЃСє1ЮЛаЁЪ§]ЁЃ
ЂлЯТЭМЗжБ№ДњБэьЪБфЃЈЁїHЃЉЁЂЛьКЯЦјЬхЦНОљЯрЖдЗжзгЃЈ![]() ЃЉЁЂSO2 жЪСПЗжЪ§[Із(SO2)]КЭЛьКЯЦјЬхбЙЧП(p)гыЗДгІЪБМф(t)ЕФЙиЯЕЃЌЯТЭМе§ШЗЧвФмБэУїИУЗДгІДяЕНЦНКтзДЬЌЕФЪЧ______________ЁЃ
ЃЉЁЂSO2 жЪСПЗжЪ§[Із(SO2)]КЭЛьКЯЦјЬхбЙЧП(p)гыЗДгІЪБМф(t)ЕФЙиЯЕЃЌЯТЭМе§ШЗЧвФмБэУїИУЗДгІДяЕНЦНКтзДЬЌЕФЪЧ______________ЁЃ
A  B
B  C
C  D
D 
Ђм40minЪБЃЌИФБфЕФЬѕМўПЩФмЪЧ_______________________________ЃЈаД2ЕуЃЉЁЃ
ЃЈ3ЃЉФГКЌюмДпЛЏМСПЩвдДпˏߞç¸гЭГЕЮВЦјжаЕФЬМбЬЃЈCЃЉКЭNOxЁЃВЛЭЌЮТЖШЯТЃЌНЋФЃФтЮВЦјЃЈГЩЗжШчЯТБэЫљЪОЃЉвдЯрЭЌЕФСїЫйЭЈЙ§ИУДпЛЏМСЃЌВтЕУЫљгаВњЮяЃЈCO2ЁЂN2ЁЂN2OЃЉгыNOЕФЯрЙиЪ§ОнНсЙЙШчЭМЫљЪОЁЃ
ФЃФтЮВЦј | ЦјЬхЃЈ10 molЃЉ | ЬМбЬ | ||
NO | O2 | He | ||
ЮяжЪЕФСП/mol | 0.025 | 0.5 | 9.475 | n |
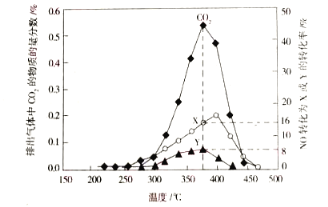
ЂйЖўбѕЛЏЬМЕФЕчзгЪНЮЊ__________________ЁЃ
Ђк375ЁцЪБЃЌВтЕУХХГіЕФЦјЬхжаКЌ0.45molO2КЭ0.0525molCO2ЃЌдђYЕФЛЏбЇУћГЦЮЊ______ЁЃ
ЁОД№АИЁПC2H2(g)+5/2 O2(g)= CO2(g)+ H2O(l) ЁїH=(2ЁїH2-ЁїH1+ЁїH3) 60% 0.8 BC Щ§ИпЮТЖШЁЂдіМгЗДгІЮяЛђЩњГЩЮяЕФЮяжЪЕФСП ![]() вЛбѕЛЏЖўЕЊ
вЛбѕЛЏЖўЕЊ
ЁОНтЮіЁП
ЃЈ1ЃЉвбжЊШШЛЏбЇЗНГЬЪНЃКЂй2C(s)+H2(g) = C2H2(g)ЁїH1ЃЛЂкC(s)+O2(g) = CO2(g)ЁїH2ЃЛЂлH2(g) +![]() O2(g) = H2O(l) ЁїH3ЃЌИљОнИЧЫЙЖЈТЩЂк
O2(g) = H2O(l) ЁїH3ЃЌИљОнИЧЫЙЖЈТЩЂк![]() 2-Ђй+ЂлЮЊC2H2(g)+5/2 O2(g)= CO2(g)+ H2O(l) ЁїH=(2ЁїH2-ЁїH1+ЁїH3)ЁЃC2H2ШМЩеШШЕФШШЛЏбЇЗНГЬЪНЮЊC2H2(g)+5/2 O2(g)= CO2(g)+ H2O(l) ЁїH=(2ЁїH2-ЁїH1+ЁїH3)ЁЃД№АИЃКC2H2(g)+5/2 O2(g)= CO2(g)+ H2O(l) ЁїH=(2ЁїH2-ЁїH1+ЁїH3)ЁЃ
2-Ђй+ЂлЮЊC2H2(g)+5/2 O2(g)= CO2(g)+ H2O(l) ЁїH=(2ЁїH2-ЁїH1+ЁїH3)ЁЃC2H2ШМЩеШШЕФШШЛЏбЇЗНГЬЪНЮЊC2H2(g)+5/2 O2(g)= CO2(g)+ H2O(l) ЁїH=(2ЁїH2-ЁїH1+ЁїH3)ЁЃД№АИЃКC2H2(g)+5/2 O2(g)= CO2(g)+ H2O(l) ЁїH=(2ЁїH2-ЁїH1+ЁїH3)ЁЃ
ЃЈ2ЃЉгЩБэИёЪ§ОнПЩжЊ15minЪБДяЕНЦНКтЃЌРћгУШ§ЖЮЪН
2SO2 (g) +O2(g) ![]() 2 SO3(g)
2 SO3(g)
ГѕЪМСПЃЈmolЃЉ 2 1 0
БфЛЏСПЃЈmolЃЉ 2x x 2x
ЦНКтСПЃЈmolЃЉ2-2x 1- x 2x
ИљОнбЙЧПжЎБШЕШгкЮяжЪЕФСПжЎБШЃК20.0/16.0=3/(3-x).НтЕУx=0.6mol, ЂйЦНКтЪБЃЌSO2ЕФзЊЛЏТЪІС=0.6![]() /2=60%ЃЛД№АИЃК60%ЁЃ
/2=60%ЃЛД№АИЃК60%ЁЃ
ЂкTЁцЪБЃЌЗДгІ2SO2 (g) +O2(g) ![]() 2 SO3(g)ЕФЦНКтГЃЪ§Kp=P2(SO3 )/[p2 (SO2)p(O2)]=( 16.0
2 SO3(g)ЕФЦНКтГЃЪ§Kp=P2(SO3 )/[p2 (SO2)p(O2)]=( 16.0![]() 1.2/2.4)2/[(16.0
1.2/2.4)2/[(16.0![]() 0.8/2.4)2
0.8/2.4)2![]() ( 16.0
( 16.0![]() 0.4/2.4)]=0.8kPaЃ1ЁЃД№АИЃК0.8
0.4/2.4)]=0.8kPaЃ1ЁЃД№АИЃК0.8
ЂлвђЮЊЛЏбЇЗДгІАщЫцФмСПБфЛЏЃЌЫљвдЕБьЪБфВЛдкБфЛЏЪБЃЌЫЕУїЛЏбЇЗДгІДяЕНЦНКтзДЬЌЃЌЙЪAДэЮѓЃЛ
вђЮЊ2SO2 (g) +O2(g) ![]() 2 SO3(g)ЪЧСНБпЛЏбЇМЦСПЪ§ВЛЕШЧвЪЧЦјЬхЗжзгЪ§МѕаЁЕФЗДгІЃЌЫљвдЫцЗДгІНјааЃЌЛьКЯЦјЬхЦНОљЯрЖдЗжзгжЪСПж№НЅдіДѓЃЌЕБЛьКЯЦјЬхЦНОљЯрЖдЗжзгжЪСПВЛдкБфЛЏЫЕУїЛЏбЇЗДгІДяЕНЦНКтзДЬЌЃЌЙЪBе§ШЗЃЛ
2 SO3(g)ЪЧСНБпЛЏбЇМЦСПЪ§ВЛЕШЧвЪЧЦјЬхЗжзгЪ§МѕаЁЕФЗДгІЃЌЫљвдЫцЗДгІНјааЃЌЛьКЯЦјЬхЦНОљЯрЖдЗжзгжЪСПж№НЅдіДѓЃЌЕБЛьКЯЦјЬхЦНОљЯрЖдЗжзгжЪСПВЛдкБфЛЏЫЕУїЛЏбЇЗДгІДяЕНЦНКтзДЬЌЃЌЙЪBе§ШЗЃЛ
SO2ЪєгкЗДгІЮяЃЌЫцЗДгІНјааЃЌSO2 жЪСПЗжЪ§ж№НЅМѕаЁЃЌЕБSO2 жЪСПЗжЪ§ВЛдкБфЛЏЫЕУїЗДгІДяЕНЦНКтзДЬЌЃЌЙЪCе§ШЗЃЛ
вђЮЊ2SO2 (g) +O2(g) ![]() 2 SO3(g)ЪЧЦјЬхЗжзгЪ§МѕаЁЕФЗДгІЃЌЫцзХЗДгІНјааЃЌбЙЧПж№НЅМѕаЁЃЌЕБбЙЧПВЛдкИФБфЪЧЃЌЗДгІДяЕНЦНКтзДЬЌЃЌЙЪDДэЮѓЃЛД№АИЃКBCЁЃ
2 SO3(g)ЪЧЦјЬхЗжзгЪ§МѕаЁЕФЗДгІЃЌЫцзХЗДгІНјааЃЌбЙЧПж№НЅМѕаЁЃЌЕБбЙЧПВЛдкИФБфЪЧЃЌЗДгІДяЕНЦНКтзДЬЌЃЌЙЪDДэЮѓЃЛД№АИЃКBCЁЃ
Ђм40minЪБЃЌИеадШнЦїжаЦјЬхЕФбЙЧПдіДѓЁЃИљОнРэЯыЦјЬхЕФзДЬЌЗНГЬPV=nRTПЩжЊЃЌЩ§ИпЮТЖШПЩвддіДѓЬхЯЕЕФбЙЧПЃЌЖјЧвЩ§ИпЮТЖШПЩвдЪЙЛЏбЇЦНКтЯђФцЗДгІЗНЯђвЦЖЏЃЌЬхЯЕЕФбЙЧПвВдіДѓЁЃЕБШЛЃЌжБНгдіМгЦјЬхЕФЮяжЪЕФСПвВФмдіДѓЬхЯЕЕФбЙЧПЁЃЙЪИФБфЕФЬѕМўПЩЪЧЩ§ИпЮТЖШЃЌвВПЩФмЪЧдіМгЗДгІЮяЛђЩњГЩЮяЕФЮяжЪЕФСПЁЃД№АИЃКЩ§ИпЮТЖШЁЂдіМгЗДгІЮяЛђЩњГЩЮяЕФЮяжЪЕФСПЁЃ
ЃЈ3ЃЉЂйЖўбѕЛЏЬМЕФЗжзгЪНЮЊCO2,ЪєгкЙВМлЛЏКЯЮяЃЌЕчзгЪНЮЊД№АИЃК![]() ЁЃ
ЁЃ
ЂкФЃФтЮВЦјжагаNO 0.025 mol,гЩЭМПЩжЊ,ЦфжаВЮгыЗДгІЕФNOЕФЮяжЪЕФзюЮЊ0. 025 mol![]() (8%+16%)= 0. 006 mol ,ФЃФтЮВЦјжагаO20.5 mol ,ХХГіЦјЬхжагаO2 ЮЊ0.45 mol, ЙЪЪЕМЪВЮМгЗДгІЕФO2ЮЊ0. 05 mol ЁЃХХГіЕФЦјЬхжага0. 0525 mol CO2ЃЌИљОнбѕдЊЫиЪиКуЃЌПЩжЊN2OЕФЮяжЪЕФСПЮЊ0.05 mol
(8%+16%)= 0. 006 mol ,ФЃФтЮВЦјжагаO20.5 mol ,ХХГіЦјЬхжагаO2 ЮЊ0.45 mol, ЙЪЪЕМЪВЮМгЗДгІЕФO2ЮЊ0. 05 mol ЁЃХХГіЕФЦјЬхжага0. 0525 mol CO2ЃЌИљОнбѕдЊЫиЪиКуЃЌПЩжЊN2OЕФЮяжЪЕФСПЮЊ0.05 mol![]() 2+0. 006 mol-0. 0525 mol
2+0. 006 mol-0. 0525 mol![]() 2=0.001 mol,ИљОнЕЊдЊЫиЪиКу,ПЩжЊn(N2 )=(0.006 mol-0.001 mol
2=0.001 mol,ИљОнЕЊдЊЫиЪиКу,ПЩжЊn(N2 )=(0.006 mol-0.001 mol![]() 2)/2 =0.002molЃЌвђДЫЃЌNOзЊЛЏЮЊвЛбѕЛЏЖўЕЊЕФзЊЛЏТЪНЯЕЭЃЌЫљвдXЮЊN2, YЮЊN2OЃЛД№АИЃКвЛбѕЛЏЖўЕЊЁЃ
2)/2 =0.002molЃЌвђДЫЃЌNOзЊЛЏЮЊвЛбѕЛЏЖўЕЊЕФзЊЛЏТЪНЯЕЭЃЌЫљвдXЮЊN2, YЮЊN2OЃЛД№АИЃКвЛбѕЛЏЖўЕЊЁЃ

ЁОЬтФПЁПЂіAзхдЊЫиаЮГЩЕФЛЏКЯЮядкЪЕбщЪвКЭЙЄвЕЩњВњЩЯгазХЙуЗКЕФгІгУЁЃЛиД№ЯТСаЮЪЬтЃК
(1)SCN-гыFe3ЃЋПЩаЮГЩЖржжХфРызгЃЌЦфжавЛжжЮЊ[Fe(SCN)6]3-ЃЌИУХфРызгжаЕФSCN-ЛсЪЙFe3ЃЋЕФЪЃгрМлЕчзгбЙЫѕХфЖдЃЌдђУПИіХфРызгжаFe3ЃЋЕФЕЅЕчзгИіЪ§ЮЊ_________ИіЁЃ
(2)SeгыSЪЧЭЌзхдЊЫиЃЌЧыаДГіЛљЬЌSeдзгЕчзгХХВМЪН_____________ЁЃH2SeЕФЫсадБШH2S________(ЬюЁАЧПЁБЛђЁАШѕЁБ)ЁЃH2OЁЂH2SЁЂH2SeЗаЕугЩИпЕНЕЭЕФЫГађЮЊ__________________ЃЌдвђЪЧЃК___________ЁЃ
(3)гавЛжжгЩ1ЁЋ9КХдЊЫижаЕФВПЗждЊЫизщГЩЃЌЧвгыSCl2ЛЅЮЊЕШЕчзгЬхЕФЙВМлЛЏКЯЮяЃЌЫќЕФЗжзгЪНЮЊ_______ЃЌЦфVSEPRЙЙаЭЮЊ_____________ЁЃ
(4)вбжЊS4O62-ЕФНсЙЙЮЊ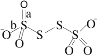 ЃЌЦфжаSдзгЕФдгЛЏЗНЪНЪЧ_______________ЁЃМќГЄa______b(ЬюЁАЃОЁБЁЂЁАЃМЁБЛђЁАЃНЁБ)ЁЃ
ЃЌЦфжаSдзгЕФдгЛЏЗНЪНЪЧ_______________ЁЃМќГЄa______b(ЬюЁАЃОЁБЁЂЁАЃМЁБЛђЁАЃНЁБ)ЁЃ
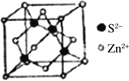
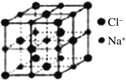
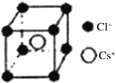
(5)РызгОЇЬхжабєРызгКЭвѕРызгЕФАыОЖБШВЛЭЌПЩаЮГЩВЛЭЌЕФОЇАћНсЙЙЃЌМћЯТБэЃК
АыОЖБШ | 0.225ЁЋ0.414 | 0.414ЁЋ0.732 | 0.732ЁЋ1 |
ЕфаЭЛЏбЇЪН | СЂЗНZnS | NaCl | CsCl |
ОЇАћ |
|
|
|
вбжЊФГРызгОЇЬхRAЃЌЦфвѕбєРызгАыОЖЗжБ№ЮЊ184pmКЭ74pmЃЌФІЖћжЪСПЮЊM g/molЃЌдђбєРызгХфЮЛЪ§ЮЊ___________ЃЌОЇЬхЕФУмЖШЮЊ_________g/cm3(СаГіМЦЫуЪНЃЌЮоашЛЏМђЃЌЩшNAЮЊАЂЗќМгЕТТоГЃЪ§ЕФжЕ)ЁЃ
ЁОЬтФПЁПЃЈ1ЃЉвбжЊHЁЊHМќМќФм(ЛЏбЇМќЖЯСбЪБЮќЪеЛђаЮГЩЪБЪЭЗХЕФФмСП)ЮЊ436 kJ/molЃЌNЁЊHМќМќФмЮЊ391 kJ/molЃЌИљОнШШЛЏбЇЗНГЬЪНЃКN2(g)ЃЋ3H2(g)===2NH3(g)ЁЁІЄHЃНЃ92.4 kJ/molЃЌПЩжЊ![]() МќЕФМќФмЪЧ______________kJ/mol
МќЕФМќФмЪЧ______________kJ/mol
ЃЈ2ЃЉЬМ(s)дкбѕЦјЙЉгІВЛГфзуЪБЃЌЩњГЩCOЭЌЪБЛЙВПЗжЩњГЩCO2ЃЌвђДЫЮоЗЈЭЈЙ§ЪЕбщжБНгВтЕУЗДгІЃКC(s)ЃЋ![]() O2(g)===CO(g)ЕФІЄHЁЃЕЋПЩЩшМЦЪЕбщЁЂРћгУИЧЫЙЖЈТЩМЦЫуГіИУЗДгІЕФІЄHЃЌМЦЫуЪБашвЊВтЕУЕФЪЕбщЪ§Онга________
O2(g)===CO(g)ЕФІЄHЁЃЕЋПЩЩшМЦЪЕбщЁЂРћгУИЧЫЙЖЈТЩМЦЫуГіИУЗДгІЕФІЄHЃЌМЦЫуЪБашвЊВтЕУЕФЪЕбщЪ§Онга________
ЃЈ3ЃЉдЫЖЏЛсжаЕФЛ№ОцвЛАуВЩгУБћЭщ(C3H8)ЮЊШМСЯЁЃБћЭщШШжЕНЯИпЃЌЮлШОНЯаЁЃЌЪЧвЛжжгХСМЕФШМСЯЁЃЪдЛиД№ЯТСаЮЪЬтЃК
ЂйШчЭМЪЧвЛЖЈСПБћЭщЭъШЋШМЩеЩњГЩCO2КЭ1 mol H2O(l)Й§ГЬжаЕФФмСПБфЛЏЭМЃЌЭМжаЕФРЈКХФкгІЬюШы___ЃЈЁАЃЋЁБЛђЁАЃЁБЃЉЁЃ

ЂкаДГіБэЪОБћЭщШМЩеШШЕФШШЛЏбЇЗНГЬЪНЃК________________ЁЃ
ЃЈ4ЃЉИпТШЫсЁЂСђЫсЁЂЯѕЫсКЭбЮЫсЖМЪЧЧПЫс,ЦфЫсаддкЫЎШмвКжаВюБ№ВЛДѓЁЃвдЯТЪЧФГЮТЖШЯТетЫФжжЫсдкБљДзЫсжаЕФЕчРыГЃЪ§:
Ыс | HClO4 | H2SO4 | HCl | HNO3 |
Ka | 1.6ЁС10-5 | 6.3ЁС10-9 | 1.6ЁС10-9 | 4.2ЁС10-10 |
гЩвдЩЯБэИёжаЪ§ОнХаЖЯвдЯТЫЕЗЈВЛе§ШЗЕФЪЧ__________ЁЃ
A.дкБљДзЫсжаетЫФжжЫсЖМУЛгаЭъШЋЕчРы
B.дкБљДзЫсжаИпТШЫсЪЧетЫФжжЫсжаЫсадзюЧПЕФЫс
C.дкБљДзЫсжаСђЫсЕФЕчРыЗНГЬЪНЮЊ: H2SO4 = 2H++S![]()
D.ЫЎЖдетЫФжжЫсЕФЧПШѕУЛгаЧјЗжФмСІ,ЕЋБљДзЫсПЩвдЧјЗжетЫФжжЫсЕФЧПШѕ
ЃЈ5ЃЉГЃЮТЯТ,0.1 molЁЄL-1ЕФCH3COOHШмвКМгЫЎЯЁЪЭЙ§ГЬжа,ЯТСаБэДяЪНЕФЪ§жЕБфДѓЕФЪЧ________ЁЃ
A.c(H+)ЁЁЁЁB.![]() ЁЁЁЁC.c(CH3COO-)ЁЁЁЁD.c(CH3COOH)
ЁЁЁЁC.c(CH3COO-)ЁЁЁЁD.c(CH3COOH)
ЁОЬтФПЁПЯжгаЪвЮТЯТЫФжжШмвКЃЌгаЙиа№ЪіВЛе§ШЗЕФЪЧ
БрКХ | Ђй | Ђк | Ђл | Ђм |
pH | 10 | 10 | 4 | 4 |
ШмвК | АБЫЎ | ЧтбѕЛЏФЦШмвК | ДзЫсШмвК | бЮЫс |
A. ЯрЭЌЬхЛ§ЂлЁЂЂмШмвКЗжБ№гыNaOHЭъШЋЗДгІЃЌЯћКФNaOHЮяжЪЕФСПЃКЂлЃОЂм
B. ЗжБ№МгЫЎЯЁЪЭ10БЖЃЌЫФжжШмвКЕФpHЃКЂйЃОЂкЃОЂмЃОЂл
C. ЂйЁЂЂмСНШмвКЕШЬхЛ§ЛьКЯЃЌЫљЕУШмвКжаcЃЈNH4ЃЋЃЉЃОcЃЈClЃЃЉЃОcЃЈOHЃЃЉЃОcЃЈHЃЋЃЉ
D. VaLЂмШмвКгыVbLЂкШмвКЛьКЯЃЈНќЫЦШЯЮЊЛьКЯШмвКЬхЛ§ЃНVaЃЋVbЃЉЃЌШєЛьКЯКѓШмвКpHЃН5ЃЌдђVaЉUVbЃН9ЉU11