��Ŀ����
����Ŀ��A��B��C��D����ѧ��ѧ�����ĵ��ʣ��ס��ҡ���Ϊ��������ڳ��³�ѹ��Ϊ��ɫ��ζ��Һ�壬��ɵ���A��Ԫ���пɱ仯�ϼۡ�����֮���������ͼ��ʾ��ת����ϵ���ɴ��ƶϣ�

(1)�Ļ�ѧʽΪ________________�����Ļ�ѧʽΪ________________��
(2)д����D��Ӧ�Ļ�ѧ����ʽ��_________________________________________��
(3)Ϊ���黯�������Ԫ�صĻ��ϼۣ���Ҫ�õ���ҩƷ��________(����ĸ)��
A.ϡ���� B.����
C.���軯����Һ D.���������Һ
���𰸡�Fe3O4 Al2O3 3Fe3O4��8Al![]() 9Fe��4Al2O3 ACD
9Fe��4Al2O3 ACD
��������
A��B��C��D����ѧ��ѧ�����ĵ��ʣ��ס��ҡ���Ϊ��������ڳ��³�ѹ��Ϊ��ɫ��ζ��Һ�壬����H2O����ɵ���A��Ԫ���пɱ仯�ϼۣ��Ҹ�����������H2O��Ӧ����A��Fe������Fe3O4��C��H2��B��O2��Fe3O4��D��Ӧ����Fe���˷�ӦΪ���ȷ�Ӧ����D��Al������Al2O3��
��1�����ڳ��³�ѹ��Ϊ��ɫ��ζ��Һ�壬����H2O����ɵ���A��Ԫ���пɱ仯�ϼۣ��Ҹ�����������H2O��Ӧ����A��Fe������Fe3O4��Fe3O4��D��Ӧ����Fe���˷�ӦΪ���ȷ�Ӧ����D��Al������Al2O3���ʼĻ�ѧʽΪFe3O4�����Ļ�ѧʽΪAl2O3��
��2������Fe3O4��D��Al���仯ѧ��Ӧ����ʽΪ��3Fe3O4��8Al![]() 9Fe��4Al2O3
9Fe��4Al2O3
��3������Fe3O4��Ҫ�������������е������ӣ�Ҫ����ϡ����ʹ�䷢����Ӧ��Fe3O4��8H����2Fe3����Fe2����4H2O��Ȼ�����ø��������Һ����Fe2���������軯����Һ����Fe3������ʹ�õ�������A��C��D��

 ��ͼͼ�麮����ҵ������ҵ���ִ�ѧ������ϵ�д�
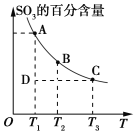
��ͼͼ�麮����ҵ������ҵ���ִ�ѧ������ϵ�д�����Ŀ��ij���жԴ������м�⣬���ָ�����Ҫ��Ⱦ��Ϊ�����������PM2.5(ֱ��С�ڵ���2.5��m������������)������Ҫ��ԴΪȼú��������β���ȡ���ˣ���PM2.5��SO2��NOx�Ƚ����о�������Ҫ���塣��ش��������⣺
(1)PM2.5��ɢ�ڿ������γɵķ�ɢϵ________(����ڡ������ڡ�)���塣
(2)��PM2.5����������ˮ�����Ƴɴ�������������ø���������ˮ���������ӵĻ�ѧ��ּ���ƽ��Ũ�����±���
���� | K�� | Na�� | NH4+ | SO42- | NO3- | Cl�� |
Ũ��/ mol��L��1 | 4��10��6 | 6��10��6 | 2��10��5 | 4��10��5 | 3��10��5 | 2��10��5 |
���ݱ��������жϴ�������Ϊ________(��ᡱ�)�ԣ���ʾ����������Ե�c(H��)��c(OH��)��________mol��L��1��
(3)Ϊ����SO2���ŷţ�����ijЩ��Һϴ�Ӻ�SO2���������������ʿ���ϴ�Ӽ�����__________________________(����ĸ)��
a.Ca(OH)2����b.Na2CO3����c.CaCl2����d.NaHSO3
(4)����β����NOx��CO�����ɼ�ת����
�����������������¶�Խ�ߣ���λʱ����NO�ŷ���Խ��д������������NO�Ļ�ѧ����ʽ��_____________________________��
������ȼ�Ͳ���ȫȼ��ʱ����CO��Ŀǰ��������β��ϵͳ��װ�ô�ת�����ɼ���CO��NO����Ⱦ���仯ѧ��Ӧ����ʽΪ__________________________��