��Ŀ����
����Ŀ����ͼ�������Լ�ƿ��ǩ�ϵIJ������ݡ��ݴ�����˵������ȷ����(����)
���ᡡ��ѧ��CP
����500 mL
Ʒ��������
��ѧʽ��H2SO4
��Է���������98
�ܶȣ�1.84 g��cm��3
����������98%
A.�������������������������
B.1molZn�������ĸ����ᷴӦ�ܲ���2gH2
C.����250mL4.6mol��L��1��ϡ������ȡ������62.5mL
D.����С�Ľ������ὦ��Ƥ���ϣ�Ӧ������NaOH��Һ��ϴ
���𰸡�C
��������
A���Լ�ƿ����ʢ��Ũ����ʱ��Ũ������ǿ�����ԣ����ܸ����������壬�����ⲻ����A����
B��Zn��Ũ���ᷴӦ���ɶ����������壬�����������������ⲻ����B����
C������250mL4.6mol��L��1��ϡ������ȡ����������ΪV=![]() =62.5mL���������⣬C��ȷ��
=62.5mL���������⣬C��ȷ��
D��Ũ������������ƶ�����ǿ�ҵĸ�ʴ�ԣ������ⲻ����D����
��ΪC��

����Ŀ��������һ�ֹ��ϵĸ���ֵ�����Դ��Ŀǰ��������������ڽ�����Ⱦ���ŷŵ�̼��Դ���ɳ�����Ч���ŷŵ�����Դ�����ܿ������������������ڣ�
һ����ȼ�ϵ��Ʊ�
(1)�ҹ���������Ҫ��Դ�ǽ�¯�����⣬���Ƶõ��������н϶��CO��H2S�����¸ɷ�������������������Ԫ��ת��Ϊ�������ɷ�����ķ�Ӧ����ʽΪ________��
(2)�ҹ�������Ա��ľмˮ����������ȡ��ȼ�ϣ���һ�������£���Ӧ���д������·�Ӧ��
i.CO2 (g) +C(s)=2CO (g) ��H1
ii.C(s) + H2O(g)=CO ( g) + H2 (g) ��H2
iii.C(s) + 2H2 (g)=CH4( g) ��H3
iv.CO(g) + H2O(g)=CO2 ( g) + H2 (g) ��H4
v. CH4(g)+ 2 H2O(g)= CO2 ( g) + 4H2 (g) ��H5
�١�H5=_________________ ��
���о��� CaO ������������CaO ��������Ca ����ľм����̼�����ʵ�����ȷ������750�棬����ˮ��������Ϊ 0.lg/(ming)�£�̽�������������Բ�����ֺ��������ܵ�Ӱ�죬��������ʾ��
n(Ca)/n(C) | ����������� | ̼ת����/% | |||
H2 | CO | CO2 | CH4 | ||
0 | 45.58 | 22.70 | 22.37 | 7.54 | 61.22 |
0.5 | 52.95 | 21.74 | 19.11 | 5.14 | 56.59 |
1.0 | 58.62 | 22.37 | 12.60 | 5.31 | 61.42 |
�ɱ������ݣ�n(Ca) / n(C) =___ʱ��Ϊ������ n(Ca) / n(C) ��0��0.5ʱ��H2����������������ӵ�ԭ��_____����ϵ�������¶Ȳ�����ľм���Ƚ�������Ӧ��Ӱ�� �����Ҷ� CaO ���� CO2 �������Լ� CaCO3�ķֽⷴӦҲ�кܴ�Ӱ�졣ʵ������У����� n(Ca) / n(C) Ϊ 1.0��ˮ��������Ϊ0.1 g/(min g)�� ��������Ӧ�¶ȴ�700������850�棬�����¶ȶԲ����ʡ������ʵ�Ӱ�������
�¶�/�� | ����������� | ̼ת����/% | ||
H2 | CO | CO2 | ||
700 | 51.78 | 20.75 | 19.89 | 54.37 |
750 | 58.62 | 22.37 | 12.60 | 61.42 |
800 | 55.63 | 26.05 | 12.71 | 73.43 |
850 | 54.16 | 26.94 | 13.82 | 83.34 |
�۴Ӳ����ʵĽǶȿ��ǣ���Ѳ����¶���________________��
�����ŷ�Ӧ�Ľ��У����� CaO �������������ͣ�ԭ����___________��
������ȼ�ϵĴ洢
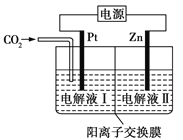
(3)������������Һ��ȼ���У����Խ�������İ�ȫ��Ч�洢���������⡣���ڼ״����е�λ����������ߡ���¶ȵ͵��ŵ㣬�������Һ�崢��ƽ̨���ӡ��ҹ�ѧ�߹���һ��˫���ܽṹ�Ĵ�������Ӧ�����У��ڴ����ı���ͬʱ�ˮ�ͼ״�����ͼ�Ǽ״�����ת���ķ�Ӧ����( TS ��ʾ����̬����
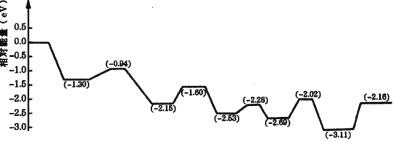
����ͼ���жϼ״����ⷴӦ�ж��ѵĻ�ѧ����______���÷�Ӧ�ġ�H___0( ������������������������С����)
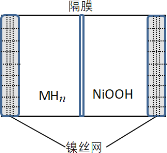
������ȼ�ϵ�Ӧ�á�һ�����ܵ�ص�ԭ����ͼ��
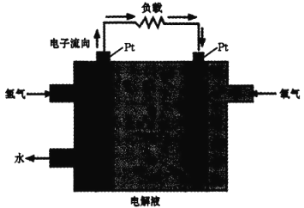
(4)�����ĵ缫��ӦʽΪ________���� Pt �缫���澵һ��ϸС��Ǧ�ۣ�ԭ����______��
����Ŀ��������Ҫ�İ뵼�����֮һ��̼���衢��λ��ͬ���塣
��1����̬��ԭ�ӵļ۲�����Ų�ʽΪ__________________��
��2�����־�����۵��Ӳ�������ʾ��
���� | ���ʯ | ̼���� | �������� | �� | �� |
�۵�/�� | 3 550 | 2 700 | 1 710 | 1 410 | 1 211 |
Ӳ�� | 10 | 9.5 | 7 | 6.5 | 6.0 |
��̼����ľ���������__________________��
������������۵�ܸߣ����ɱ�(���������̼)������������Ҫԭ����__________________��
������۵������ģ�����Ҫԭ����___________________________��
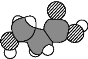
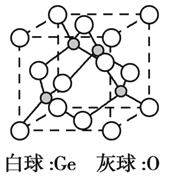
��3��![]() �����幹����________________��GeCl4����������ӻ�������__________________��
�����幹����________________��GeCl4����������ӻ�������__________________��
��4��1��CS2�����к�_________��������
��5����������ᄃ����ͼ��ʾ����֪�����������Ħ������ΪM g��mol1��NA���������ӵ�������ֵ�������ܶ�Ϊ �� g��cm3���þ����Ļ�ѧʽΪ_________����ԭ�ӵ���λ��Ϊ_________���þ�������Ϊ_________cm(�ô���ʽ��ʾ)��
 ��
��