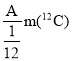��Ŀ����
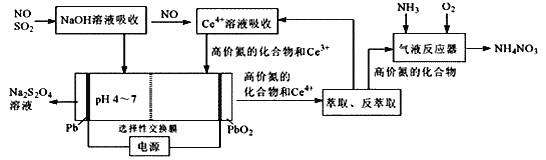
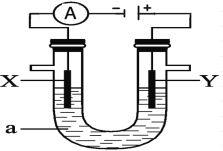
����Ŀ�����ʵ����ͺ���Ԫ�صĻ��ϼ����о��������ʵ����������ӽǡ��������ͼ��ʾ���ش��������⣺

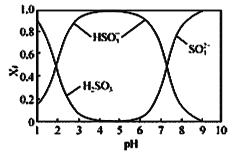
��1��Y���γ��������Ҫ����֮һ��������ˮ��Ӧ�Ļ�ѧ����ʽΪ_____����һ��������������Ҫ������������NOx��ɵģ�д��NO2��ˮ��Ӧ�Ļ�ѧ����ʽΪ______�������pH______(�Χ)��
��2��Y��ʹƷ����Һ��ɫ������һ�ֻ���ɫ��������ͨ��ˮ�еõ�����Һȴ����û��Ư���ԣ���Ӧ�����ӷ���ʽΪ_______��YҲ��ʹ���Ը��������Һ��ɫ��д���÷�Ӧ�����ӷ���ʽ______�����õ����ŷ���������ת�Ƶķ������Ŀ��������Y��_____�ԡ�
��3��W��Ũ��Һ��ͭ�����ڼ��������¿��Է�����ѧ��Ӧ����Ӧ�Ļ�ѧ����ʽΪ_____��
��4������98%��Ũ���ᣨ��=1.84g/cm3�����Ƴ�Ũ��Ϊ0.5mol/L��ϡ����500mL���ش��������⣺
������Ũ��������Ϊ______mL��
�ڽ�Ũ�������ձ��ڱڻ���ע��ʢˮ���ձ��У����Ͻ����Ŀ����_____���������������Һ�彦�����ᵼ������������ҺŨ��_____���ƫ����ƫС������Ӱ�족����ͬ����
����ת������ƿǰ���ձ��е�Һ��Ӧ��_____�������ʹŨ��_____��
��5����֪S�������Խ������������ͭ���ȷ�Ӧ�Ļ�ѧ����ʽΪ_____��
��6�����Ʊ�Na2S2O3����������ƣ�����������ԭ��Ӧ�Ƕȷ�������������_____������ţ���
A��Na2S+S B��Na2SO3+S C��Na2SO3+Na2SO4 D��SO2+Na2SO4
���𰸡�SO2+H2O=H2SO3 3NO2+H2O=2HNO3+NO ��5.6(��С��5.6) ![]()
 ��ԭ Cu+2H2SO4(Ũ)
��ԭ Cu+2H2SO4(Ũ)![]() CuSO4+SO2��+2H2O 13.6 ��ֹ�ֲ����ȵ�����Һ�ɽ� ƫС ��ȴ������ ƫ�� 2Cu+S
CuSO4+SO2��+2H2O 13.6 ��ֹ�ֲ����ȵ�����Һ�ɽ� ƫС ��ȴ������ ƫ�� 2Cu+S![]() Cu2S B
Cu2S B
��������
��1��+4�۵�SԪ�ض�Ӧ������ΪSO2��SO2���γ��������Ҫ����֮һ��������ˮ��Ӧ�Ļ�ѧ����ʽΪ��SO2+H2O=H2SO3��NO2��ˮ��Ӧ����HNO3��NO���䷴Ӧ����ʽΪ��3NO2+H2O=2HNO3+NO�������pH��5.6��
��2��SO2��SԪ��Ϊ�м��̬�����������Ժͻ�ԭ�ԣ�����һ�ֻ���ɫ����(��ΪCl2)�����ͨ��ˮ�з�����Ӧ��![]() ��SO2�����Ը��������Һ��Ӧ��������ء������̡����ᣬ����������ԭ��Ӧ��ʧ�����غ㡢ԭ���غ㡢����غ��֪�䷴Ӧ�����ӷ���ʽ�Լ������ű�ʾΪ��
��SO2�����Ը��������Һ��Ӧ��������ء������̡����ᣬ����������ԭ��Ӧ��ʧ�����غ㡢ԭ���غ㡢����غ��֪�䷴Ӧ�����ӷ���ʽ�Լ������ű�ʾΪ�� ��SO2�����������ֻ�ԭ�ԣ�
��SO2�����������ֻ�ԭ�ԣ�
��3��WΪH2SO4��ŨH2SO4��ͭ�����ڼ��������·�Ӧ��������ͭ����������ˮ���䷴Ӧ��ѧ����ʽΪ��Cu+2H2SO4(Ũ)![]() CuSO4+SO2��+2H2O��
CuSO4+SO2��+2H2O��
��4���ٸ���������ʵ���Ũ��![]() ��ѡ��500mL����ƿ����ȡŨ����ʹ����Ͳ������ϡ�Ͷ��ɿ�֪��18.4mol/L��V=0.5mol/L��500mL�����V=13.6mL��
��ѡ��500mL����ƿ����ȡŨ����ʹ����Ͳ������ϡ�Ͷ��ɿ�֪��18.4mol/L��V=0.5mol/L��500mL�����V=13.6mL��
��Ũ��������ˮ�����з��ȣ�����費�Ͻ��裬��Ŀ���Ƿ�ֹ�ֲ����ȵ�����Һ�ɽ����������������Һ�彦�����ᵼ��������ʧ�������Ƶ���ҺŨ��ƫС��
������ƿΪ����ʹ�������������ת������ƿǰ���ձ��е�Һ��Ӧ����ȴ�����£�����Һ�¶ȸ��ڳ��£��������������ȷ����תҺʱδ��ȴ�����£���Һ��ȴ������ʱ��������������ԭ����֪����Һ�������С����ҺŨ�Ȼ�ƫ��
��5��S�������Խ��������ܽ�ͭ����Ϊ��̬�������ͭ���ʼ��ȷ�Ӧ��������ͭ���䷴Ӧ����ʽΪ��2Cu+S![]() Cu2S��
Cu2S��
��6��Na2S2O3��SԪ�ػ��ϼ�Ϊ+2�ۣ���������ԭ�ĽǶȷ�������Ӧ����SԪ�ػ��ϼ۱���ֱ����2��С��2��A��S���ϼ۶�С��2��CD��S�Ļ��ϼ۶�����2��B�������⣬�ʴ�Ϊ��B��

 ��ʦָ����ĩ��̾�ϵ�д�
��ʦָ����ĩ��̾�ϵ�д�����Ŀ������ʵ�������õ��Լ�����������Ʒ![]() �г�װ�á�����������ʡȥ
�г�װ�á�����������ʡȥ![]() �ܹ��ﵽ��Ŀ�ĵ���
�ܹ��ﵽ��Ŀ�ĵ���
ѡ�� | Ŀ�� | �Լ� | ��������Ʒ |
A | ��֤�������������������� | �ữ��NaCl��Һ��Zn�缫��Fe�缫�����軯�� | �ձ��������������ߡ���ͷ�ι� |
B | ���ȷ�Ӧ | ������������ | ��ֽ���ƾ��ơ�ľ����ʢɳ�ӵ������� |
C | �������л��е���ϩ | ���Ը��������Һ | ���ҡ�����ƿ��ˮ�� |
D | �Ʊ��������� | �Ҵ������ᡢ����̼������Һ | ��С�Թܡ��ƾ��� |
A.AB.BC.CD.D
����Ŀ�����±���ʵ�����������Ľ��Ͳ���������![]()
ʵ����������� | ����Ľ��� | |
A | ��һƬ�������ھƾ������������գ������ۻ��������� | ���������۵��ر�� |
B | �ò������쵼�ܵ�����������ȼ���۲쵽����ʻ�ɫ | ��ͨ�����к�����Ԫ�� |
C | ��ˮ�м��� | �����˼������ʣ��� |
D | ������ı���Ũ��Һ�еμ�����������ˮ����δ�۲쵽��ɫ�������� | ���屽���ܽ��ڹ����ı����� |
A.AB.BC.CD.D