��Ŀ����
����Ŀ������ʵ�������õ��Լ�����������Ʒ![]() �г�װ�á�����������ʡȥ
�г�װ�á�����������ʡȥ![]() �ܹ��ﵽ��Ŀ�ĵ���
�ܹ��ﵽ��Ŀ�ĵ���
ѡ�� | Ŀ�� | �Լ� | ��������Ʒ |
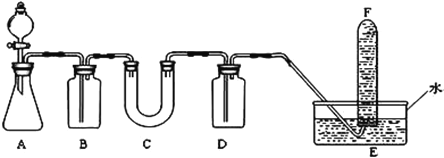
A | ��֤�������������������� | �ữ��NaCl��Һ��Zn�缫��Fe�缫�����軯�� | �ձ��������������ߡ���ͷ�ι� |
B | ���ȷ�Ӧ | ������������ | ��ֽ���ƾ��ơ�ľ����ʢɳ�ӵ������� |
C | �������л��е���ϩ | ���Ը��������Һ | ���ҡ�����ƿ��ˮ�� |
D | �Ʊ��������� | �Ҵ������ᡢ����̼������Һ | ��С�Թܡ��ƾ��� |
A.AB.BC.CD.D
���𰸡�A
��������
A.�ữNaCl��Һ��Zn�缫��Fe�缫���ɵ�ԭ����У��������軯�غ�����ɫ�������ɣ���˵��������������������A���Դﵽʵ��Ŀ�ģ���A��ȷ��
B.���ȷ�Ӧ��Ҫ���������������þ����������Ӧ����þ��������ط�Ӧ���ܷ�������B����
C.�����Ը��������Һ��ȥ��ϩʱ�������������̼�������µ����ʣ��ʲ��ܴﵽʵ��Ŀ�ģ���C����
D.������Ӧ��Ҫ��Ũ��������������ˮ������Ũ��������������ˮ������Ӧ���ܷ�������D����
��ѡA��

��ϰ��ϵ�д�
�����Ŀ