��Ŀ����
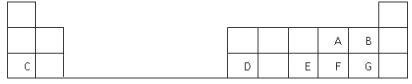
����Ŀ��NaClO2��һ��Ư�ס�������,�㷺Ӧ����ֽ�����ĵ�����Ư�ס�һ���Ʊ�NaClO2�ֲ�Ʒ�Ĺ�����������ͼ��ʾ��
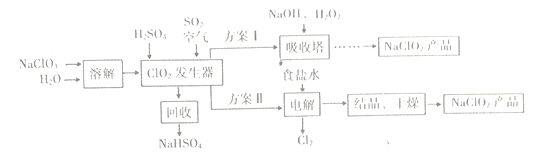
��֪:�ٴ�����ClO2���ֽ��������ը��
��NaClO2������Һ�ڵ���38��ʱ����NaClO2��3H2O����;����38��ʱ����
NaClO2����;����60��ʱNaClO2�ֽ�����NaClO3��NaCl��
�Իش��������⣺
��1���������й�����������,��Ŀ����____(ѡ�����)��
a.��SO2������SO3,����ǿ����
b.ϡ��CO2����,�Է�ֹ������ը
c.��������������ȫ������,�Լ���ClO2��ʧ
��2���������з�Ӧ�����ӷ���ʽΪ___
��3��ClO2�������з�Ӧ�Ļ�ѧ����ʽΪ___________
��4�����������пɻ�� NaClO2��Һ,��NaClO2��Һ��NaCO2��Ʒ,�����IJ�����������Ϊ:��______���¼�ѹ�����ᾧ;��________����ϴ��;�ܵ��¸���,�õ���Ʒ��
��5����������������ĵ缫��Ӧʽ��_________��������Ӧ����Ҫ������_________��
���𰸡� bc 2ClO2��H2O2��2OH�� = 2ClO2����O2��2H2O 2NaClO3��SO2��H2SO4 === 2ClO2��2NaHSO4 38�桫60�� ���� 2Cl����2e�� = Cl2�� ClO2������NaClO2��
����������1��ͨ�����̹��̿��Կ������������й���������������Ŀ����ϡ��ClO2�������Է�ֹ������ը����������������ȫ���������Լ���ClO2��ʧ�����������Ѿ����������ˣ������Ѿ���ǿ�����Խ�SO2������SO3������ǿ�����Ǵ���ģ���ȷѡ��bc��
��2��ClO2��H2O2�ڼ��Ի����£�����NaClO2��H2O2�ڸ÷�Ӧ������ԭ����������Ϊ��������Ӧ�����ӷ���ʽΪ2ClO2��H2O2��2OH�� = 2ClO2����O2��2H2O����ȷ����2ClO2��H2O2��2OH�� = 2ClO2����O2��2H2O��
��3��NaClO3����������������ԭΪClO2��SO2������Ϊ��������ӣ�����ClO2��ѧ����ʽΪ2NaClO3��SO2��H2SO4 === 2ClO2��2NaHSO4����ȷ����2NaClO3��SO2��H2SO4 === 2ClO2��2NaHSO4��
��4�����������Ϣ��NaClO2������Һ�ڵ���38��ʱ����NaClO2��3H2O����������38��ʱ������NaClO2�������60��ʱNaClO2�ֽ�����NaClO3��NaCl��֪�����������пɻ�� NaClO2��Һ����NaClO2��Һ��NaCO2��Ʒ�������IJ�����������Ϊ���ٿ���38����60���¼�ѹ�����ᾧ���ڹ�������ϴ�����ܵ��¸������õ���Ʒ����ȷ����38�桫60�� �� ������
��5����ⱥ��ʳ��ˮ���������������������ĵ缫��Ӧʽ��2Cl����2e�� = Cl2�� �� ClO2�������õ��ӻ�ԭΪClO2������NaClO2������ȷ�𰸣�2Cl����2e�� = Cl2����ClO2������NaClO2����

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ�����ᾧ�����ɿɱ�ʾΪH2C2O4��xH2O��ij�о���ѧϰС������ͼװ�ý��������ᾧ�����ȷֽ�IJ��ֲ������֤����ʵ�顣��ش��������⡣

�����ϲ��ġ�
�����ᾧ����101 ��ʱ��ʼ�ۻ���150 ��ʱ��ʼ������175 ��ʱ��ʼ�ֽ⣻
������ƺͲ�����ƾ�Ϊ��ɫ�����
��1��������ͼ��ʾ��װ����ͨ��ʵ�������ᾧ��IJ��ַֽ������װ��B�пɹ۲쵽������ð���ҳ���ʯ��ˮ��������ɴ˼�ͬѧ�жϲ��ᾧ��ֽ�IJ�������CO2�����������ͬѧ�������䷴�Ե����ɿ�����______________________________________��
��2����ͬѧ��Ϊ���ᾧ��ֽ�IJ����к���CO��Ϊ������֤��XӦѡ��________(�ѧʽ)Ũ��Һ��װ��D��������____________________��
��3��ʵ��������漰���²������ٵ�ȼװ��A���ľƾ��ƣ���Ϩ��װ��A���ľƾ��ƣ��۵�ȼװ��E���ľƾ��ƣ���Ϩ��װ��E���ľƾ��ơ���4���������ȵ����˳��Ϊ____________(�����)����ȼE���ƾ���ǰ����Ҫ���еIJ�����______________��
��4��ʵ������з���װ��E�к�ɫ��ĩ���ɫ��װ��F���к�ɫ���������������װ��F�еĹ���Ϊ������������װ��F�з�����Ӧ�Ļ�ѧ����ʽΪ________________________________________________________________________��
��5����ͬѧ�õζ����ⶨ���ᾧ���нᾧˮ�ĺ��������������в�����
����һ���÷�����ƽ��ȡ3.15 g�����ĸò��ᾧ�������Ƴ�250 mL��Һ��
�����������Һ����ȡ25.00 mL���������Һ����ƿ�������������������ữ��
��������ȡ0.100 mol��L��1������KMnO4��Һ�����еζ������ν�����±���ʾ��
��һ�� | �ڶ��� | ������ | |
������Һ���(mL) | 25.00 | 25.00 | 25.00 |
����Һ���(mL) | 9.99 | 10.01 | 10.00 |
��֪�ζ���Ӧ�����ӷ���ʽΪ��MnO![]() ��H2C2O4��H���D��Mn2����CO2����H2O(δ��ƽ)��
��H2C2O4��H���D��Mn2����CO2����H2O(δ��ƽ)��
�����Ʋ�����Һ�IJ������������ǣ������������ձ�������ˮ�ܽ�������Һת����________��ϴ�ӣ����ݣ�ҡ�ȡ�
��ͨ������ȷ��x��________��