��Ŀ����
9��A��B��C��D����Ԫ�ش���ͬһ�����ڣ���ͬ��Ԫ���У�A����̬�⻯��ķе���ߣ�B������������Ӧˮ�����������ͬ��������ǿ�ģ�C�ĵ縺�Խ���A��B֮�䣬D��B���ڣ���ش��������⣺��1����һ��B�ĵ��ʷ����д���2���м���
��2����֪B����̬�⻯���������H+��ϣ�Bԭ����H+���γɵļ���109��28�䣬�γɵ����ӵ����幹��Ϊ�������壮
��3����A��B��C��D����Ԫ���γɵĵ�������ͬ�������⻯���У��е���͵���CH4�������ʽ������е������������������⻯���ԭ���Ǽ����в��������
��4��A���⻯��������ˮ����D���⻯��������ˮ��ԭ���ǰ�����ˮ�������γ�����������ˮ���Ӳ����γ������
���� A��B��C��D����Ԫ�ش���ͬһ�����ڣ���ͬ��Ԫ���У�A����̬�⻯��ķе���ߣ���A���⻯���к���������⼸��Ԫ�ض����ڵڶ����ڣ�B������������Ӧˮ�����������ͬ��������ǿ�ģ���B��NԪ�أ�C�ĵ縺�Խ���A��B֮�䣬ͬһ����Ԫ�ص縺������ԭ�����������������C��OԪ�ء�A��FԪ�أ�
D��B���ڣ���D��CԪ�أ�
��1��B��NԪ�أ��䵥��ΪN2�����������е�ԭ��֮�����һ���Ҽ��������м���
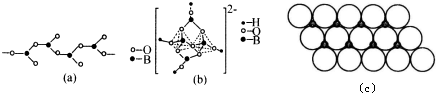
��2��BԪ���⻯���ǰ������ӣ��������Ӻ�������H+�������笠����ӣ�笠���������������ṹ��
��3���⻯���к���������۷е�ϸߣ�����������۷е�ϵͣ�
��4������������⻯����ܽ��ԣ�
��� �⣺A��B��C��D����Ԫ�ش���ͬһ�����ڣ���ͬ��Ԫ���У�A����̬�⻯��ķе���ߣ���A���⻯���к���������⼸��Ԫ�ض����ڵڶ����ڣ�B������������Ӧˮ�����������ͬ��������ǿ�ģ���B��NԪ�أ�C�ĵ縺�Խ���A��B֮�䣬ͬһ����Ԫ�ص縺������ԭ�����������������C��OԪ�ء�A��FԪ�أ�
D��B���ڣ���D��CԪ�أ�
��1��B��NԪ�أ��䵥��ΪN2�����������д��ڵ�����������ԭ��֮�����һ���Ҽ��������м����ʴ�Ϊ��2��
��2��BԪ���⻯���ǰ������ӣ��������Ӻ�������H+�������笠����ӣ��������109��28�䣬笠������е�ԭ�Ӽ۲���ӶԸ�����4�Ҳ����µ��Ӷԣ�����笠���������������ṹ��
�ʴ�Ϊ��109��28�䣻�������壻
��3���⻯���к���������۷е�ϸߣ�����������۷е�ϵͣ��⼸��Ԫ���γɵ��⻯����ֻ�м��鲻������������۷е�ϵ͵��⻯����CH4���ʴ�Ϊ��CH4�������в��������
��4������������⻯����ܽ��ԣ�A�γɵ��⻯���ǰ������������Ӻ�ˮ����֮�����γ�������Ӷ��ٽ������ܽ⣬�����ˮ���Ӳ����γ���������Լ�����ܽ��Խϵͣ��ʴ�Ϊ��������ˮ�������γ�����������ˮ���Ӳ����γ������
���� ���⿼��Ԫ��λ�ýṹ���ʹ�ϵ��Ӧ�ã��漰��������ӽṹ��֪�����Ӱ�����ʵ��۷е㡢�ܽ��Ե��������ʲ�Ӱ�컯ѧ���ʣ���Ŀ�ѶȲ���

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�| A�� | ��Ӧ������NO�����Ϊ28 L | B�� | ������Һ�е�����ֻ��FeSO4 | ||
| C�� | ������Һ��c��NO3-��=2.75 mol��L-1 | D�� | ������Һ��c��Fe2+����c��Fe3+ ��=1��1 |
| A�� | �����Ӱ뾶�Ĵ�С˳��Ϊ��rw��rz��ry | |
| B�� | Ԫ��W���������Ӧˮ��������Ա�Y��ǿ | |
| C�� | X��Y�γɵľ���XaYa���۵��Ӳ�ȴ�Ϊ��������ʯ�IJ��� | |
| D�� | X��W�γɵĻ������Z��W�γɵĻ�����Ļ�ѧ������ͬ |