��Ŀ����
14�����������ʣ���ˮ�� �ڱ����� �������� �ܰ��� �ݾ���� ���������� ���� ����ʯ ��������� ��10��̼���裨11���ɱ� ��12���������⣬����Ҫ����գ���1������ԭ�Ӿ���Ļ������Ǣ٢ࣨ10����
��2��ֱ����ԭ�ӹ��ɵķ��Ӿ����Ǣݣ�
��3���ɼ��Է��ӹ��ɵľ����Ǣڣ�12�������ڷ��Ӿ���ĵ����Ǣܢݣ�
��4����һ�������£��ܵ����Ҳ�������ѧ��Ӧ���Ǣۢޢᣬ�����ۻ���˷����ۼ����Ǣ٢ࣨ10����
���� ��1����ԭ�ӹ��ɵľ�������ԭ�Ӿ��壬ԭ�Ӿ����۷е�ϸߡ�Ӳ�Ƚϴ�
��2����ԭ�ӷ����γɵľ�����ֱ����ԭ�ӹ��ɵķ��Ӿ��壻
��3������������������IJ��غϵķ���Ϊ���Է��ӣ��ɼ��Է��ӹ��ɵľ����Ƿ��Ӿ��壻�ɷ��ӹ��ɵľ����Ƿ��Ӿ��壬������ֻ��һ��Ԫ�����Ǵ����
��4����һ�������£��ܵ����Ҳ�������ѧ��Ӧ������Ϊ���Ӿ��壻�����ۻ���Ҫ�˷����ۼ�������Ϊԭ�Ӿ��壮
��� �⣺��1����ԭ�ӹ��ɵľ�������ԭ�Ӿ��壬�⼸��������ԭ�ӹ��ɵ�Ϊ�٢ࣨ10������������ԭ�Ӿ�����Ǣ٢ࣨ10�����ʴ�Ϊ���٢ࣨ10����
��2����ԭ�ӷ����γɵľ�����ֱ����ԭ�ӹ��ɵķ��Ӿ��壬�⼸��������Ϊ��ԭ�ӷ��ӵ���Ar������ֱ���ɵ�ԭ�ӹ��ɵķ��Ӿ����Ǣݣ��ʴ�Ϊ���ݣ�
��3������������������IJ��غϵķ���Ϊ���Է��ӣ��ɼ��Է��ӹ��ɵľ����Ƿ��Ӿ��壬�ɼ��Է��ӹ��ɵķ��Ӿ���Ϊ�ڣ�12�����ɷ��ӹ��ɵľ����Ƿ��Ӿ��壬������ֻ��һ��Ԫ�����Ǵ�������ڷ��Ӿ���ĵ����Ǣܢݣ��ʴ�Ϊ���ڣ�12�����ܢݣ�
��4����һ�������£��ܵ����Ҳ�������ѧ��Ӧ������Ϊ���Ӿ��壬�������Ӿ�����Тۢޢ�����ۻ���Ҫ�˷����ۼ�������Ϊԭ�Ӿ��壬����ԭ�Ӿ����Ϊ�٢ࣨ10�������������ۻ���Ҫ�˷����ۼ���Ϊ�ڢ٢ࣨ10�����ʴ�Ϊ���ۢޢ�٢ࣨ10����
���� ���⿼�黯ѧ�����������͵��жϵ�֪ʶ�㣬��ȷ���ʹ������ǽⱾ��ؼ���ע��ԭ�Ӿ����ۻ�ʱ��Ҫ�˷����ۼ������Ӿ����ۻ�ʱ�˷����Ӽ���������ע����ߵ�������Ŀ�ѶȲ���

����˵������ȷ���ǣ�������
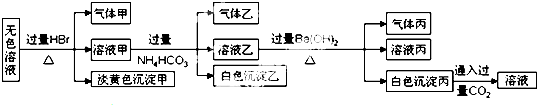
| A�� | ����ɫ�����ײ�����ΪAgBr | |
| B�� | ����Һ�����������ҵ�;��ֻ�У�Al3++3HCO3-�TAl��OH��3��+3CO2�� | |
| C�� | ��������ǻ������ | |
| D�� | �ۺ�������Ϣ����ȷ���϶����ڵ������У�Na+��AlO2-��S2O32- |
| A�� | ������pH=11�İ�ˮ��Ϻ�������ҺpHС��7 | |
| B�� | ��pH=3��CH3COOH��Һ��Ϻ�������ҺpHС��3 | |
| C�� | ���Ũ�ȵ�CH3COONa��Һ��Ϻ�������ҺpHһ��С��7 | |
| D�� | ��10L Ba��OH��2��Һǡ����ȫ��Ӧ����Ba��OH��2��Һ��pHһ������10 |
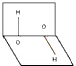
| A�� | ���ӵ���������������غ� | B�� | ����������������IJ��غ� | ||
| C�� | H2O2�Ǽ��Է��� | D�� | H2O2�ǷǼ��Է��� |
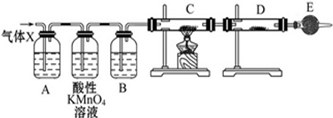
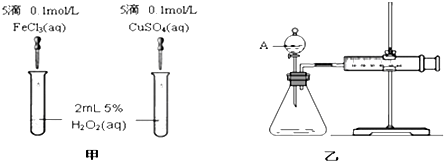
| A�� | SO2 | B�� | H2 | C�� | NH3 | D�� | CO2 |