��Ŀ����
��ͼΪʵ����ijŨ�����Լ�ƿ�ϵı�ǩ���Ը����й����ݻش��������⣺��1����Ũ������HCl�����ʵ���Ũ��Ϊ______mol/L��
��2��ȡ����������ĸ�������Һʱ�������������в�����ȡ����Ķ��ٶ��仯����______��
A����Һ��HCl�����ʵ��� B����Һ��Ũ��
C����Һ��Cl-����Ŀ D����Һ���ܶ�
��3��ijѧ����������Ũ���������ˮ����500mL���ʵ���Ũ��Ϊ0.3mol/Lϡ���ᣮ
�ٸ�ѧ����Ҫ��ȡ______ mL����Ũ����������ƣ�
�����ƹ����У���Ҫʹ�õ������ǣ���д���ţ�______��
A���ձ� B����Ͳ C��1000mL����ƿ D��������ƽ
E��ҩ�� F��500mL����ƿ G����ͷ�ι� H��������
������ʱ������ȷ�IJ���˳���ǣ���ĸ��ʾ��ÿ����ĸֻ����һ�Σ�______��
A����30mLˮϴ���ձ�2-3�Σ�ϴ��Һ��ע������ƿ����
B������Ͳȷ��ȡ����Ũ���������������ر���ע��ʢ������ˮ��Լ30mL�����ձ��У��ò���������������ʹ���Ͼ���
C��������ȴ�������ز�����ע��500mL������ƿ��
D��������ƿ�ǽ����ߵ�ҡ��
E�����ý�ͷ�ιܼ�ˮ��ʹ��Һ����ǡ����̶�������
F������������ƿ��С�ļ�ˮ��ֱ��Һ��ӽ��̶���1-2cm��
�������ƹ����У�����ʵ�������ʹ�����Ƶ�ϡ��������ʵ���Ũ��ƫ�ߵ���______
A������Ͳ��ȡŨ����ʱ���ӹ۲찼Һ��
B����Һע������ƿǰû�лָ������¾ͽ��ж���
C������ʱ���ӿ̶���
D��������ǰ����֪Ũ�ȵ�ϡ������ϴ����ƿ
��4�����ڱ�״���£���VLHCl��������1Lˮ�У�������Һ�ܶ�Ϊd g/mL�������Һ�����ʵ���Ũ��Ϊ______mol/L��
��5���ֽ�100mL0.5mol/L������200mL0.1mol/LCuCl2��Һ��ϣ�����仯���Բ��ƣ�������Һ��Cl-�����ʵ���Ũ����______��

���𰸡���������1������c=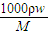 ���㣻
���㣻
��2����ҺΪ��һ�ȶ���ɢϵ��Ũ�ȡ��ܶȲ��䣻
��3���ٸ�����Һϡ��ǰ�����ʵ����ʵ������������������������
�ڸ�����Һ�����Ʋ���ѡ����ʹ�õ�������
�۲��������м��㡢�������ܽ⡢��Һ��ϴ����Һ�����ݡ�ҡ�ȵȲ������Դ��жϣ�
�ܸ���c= �������������ʵ����ʵ��������Һ�������Ӱ���жϣ�
�������������ʵ����ʵ��������Һ�������Ӱ���жϣ�
��4���ֱ�������ʵ����ʵ���������������������Һ�������������Һ�����������c=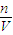 ����Ũ�ȣ�
����Ũ�ȣ�
��5�����������غ���������ӵ������ʵ�������������Ũ�ȣ�
����⣺��1��c= =
=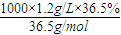 =12mol/L���ʴ�Ϊ��12mol/L��
=12mol/L���ʴ�Ϊ��12mol/L��
��2����ҺΪ��һ�ȶ���ɢϵ��Ũ�ȡ��ܶȲ��䣬�������ͬʱ�����ʵ����ʵ�����������Ŀ�仯���ʴ�Ϊ��BD��
��3��������ҪŨ��������ΪV��0.3mol/L×0.5L=12mol/L×V��V=0.0125L=12.5mL���ʴ�Ϊ��12.5��
�ڲ��������м��㡢��ȡ���ܽ⡢��Һ��ϴ����Һ�����ݡ�ҡ�ȵȲ�����һ������Ͳ�����ձ����ܽ⣨������Ͳ��ȡˮ�����ձ��������ò��������裬�����ܽ⣮��ȴ��ת�Ƶ�500mL����ƿ�У����ò�����������ϴ���ձ���������2-3�Σ�����ϴ��Һ��������ƿ�У���ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�����ݵߵ�ҡ�ȣ�����������������Ͳ���ձ�����������500mL����ƿ����ͷ�ιܣ��ʴ�Ϊ��ABFGH��
�۲��������м��㡢��ȡ��ϡ�͡���Һ��ϴ����Һ�����ݡ�ҡ�ȵȲ���������Ͳ���õ���ͷ�ιܣ�ȷ��ȡ����Ũ�������������ձ���ϡ�ͣ��ò��������裬�ָ����º�ת�Ƶ�500mL����ƿ�У����ò�����������ϴ��2-3�Σ���ϴ��Һת�Ƶ�����ƿ�У���ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�����ݵߵ�ҡ�ȣ�
�ʴ�Ϊ��BCAFED��
��A������Ͳ��ȡŨ����ʱ���ӹ۲찼Һ�棬�ᵼ����ȡҺ������ƫС��������ҺŨ��ƫС����A��ѡ��
B����Һע������ƿǰû�лָ������¾ͽ��ж��ݣ�����Һ�����ƫС����ҺŨ��ƫ��Bѡ��
C������ʱ���ӿ̶��ߣ�����Һ�����ƫС����ҺŨ��ƫ��Cѡ��
D��������ǰ����֪Ũ�ȵ�ϡ������ϴ����ƿ���ᵼ�����ʵ����ʵ���ƫ����ҺŨ��ƫ��Dѡ��
�ʴ�Ϊ��BCD��
��4��n��HCl��=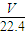 mol��m��HCl��=
mol��m��HCl��=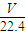 mol×36.5g/mol=
mol×36.5g/mol=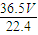 g��
g��
m����Һ��=m��HCl��+m��ˮ��=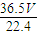 g+1000g��
g+1000g��
V����Һ��=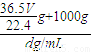 =
=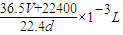 ��
��
c��HCl��=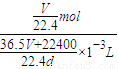 =
= mol/L��
mol/L��
�ʴ�Ϊ��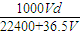 ��
��
��5��n��Cl-��=0.1L×0.5mol/L+2×0.2L×0.1mol/L=0.09mol��
V=100mL+200ml=300ml=0.3L��
c��Cl-��= =0.3mol/L��
=0.3mol/L��
�ʴ�Ϊ��0.3mol/L��
���������⿼���Ϊ�ۺϣ������Һ�����ơ�ʹ���������������衢�������Լ���ҺŨ�ȵļ��㣬��Ŀ�Ѷ��еȣ����ñ��⣬һ����Ҫ�к�ʵ�Ļ���֪ʶ�����ǰ������ʵ���Ũ�ȵļ��㷽����
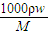 ���㣻
���㣻��2����ҺΪ��һ�ȶ���ɢϵ��Ũ�ȡ��ܶȲ��䣻
��3���ٸ�����Һϡ��ǰ�����ʵ����ʵ������������������������
�ڸ�����Һ�����Ʋ���ѡ����ʹ�õ�������
�۲��������м��㡢�������ܽ⡢��Һ��ϴ����Һ�����ݡ�ҡ�ȵȲ������Դ��жϣ�
�ܸ���c=
 �������������ʵ����ʵ��������Һ�������Ӱ���жϣ�
�������������ʵ����ʵ��������Һ�������Ӱ���жϣ���4���ֱ�������ʵ����ʵ���������������������Һ�������������Һ�����������c=
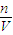 ����Ũ�ȣ�
����Ũ�ȣ���5�����������غ���������ӵ������ʵ�������������Ũ�ȣ�
����⣺��1��c=
 =
=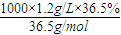 =12mol/L���ʴ�Ϊ��12mol/L��
=12mol/L���ʴ�Ϊ��12mol/L����2����ҺΪ��һ�ȶ���ɢϵ��Ũ�ȡ��ܶȲ��䣬�������ͬʱ�����ʵ����ʵ�����������Ŀ�仯���ʴ�Ϊ��BD��
��3��������ҪŨ��������ΪV��0.3mol/L×0.5L=12mol/L×V��V=0.0125L=12.5mL���ʴ�Ϊ��12.5��
�ڲ��������м��㡢��ȡ���ܽ⡢��Һ��ϴ����Һ�����ݡ�ҡ�ȵȲ�����һ������Ͳ�����ձ����ܽ⣨������Ͳ��ȡˮ�����ձ��������ò��������裬�����ܽ⣮��ȴ��ת�Ƶ�500mL����ƿ�У����ò�����������ϴ���ձ���������2-3�Σ�����ϴ��Һ��������ƿ�У���ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�����ݵߵ�ҡ�ȣ�����������������Ͳ���ձ�����������500mL����ƿ����ͷ�ιܣ��ʴ�Ϊ��ABFGH��
�۲��������м��㡢��ȡ��ϡ�͡���Һ��ϴ����Һ�����ݡ�ҡ�ȵȲ���������Ͳ���õ���ͷ�ιܣ�ȷ��ȡ����Ũ�������������ձ���ϡ�ͣ��ò��������裬�ָ����º�ת�Ƶ�500mL����ƿ�У����ò�����������ϴ��2-3�Σ���ϴ��Һת�Ƶ�����ƿ�У���ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�����ݵߵ�ҡ�ȣ�
�ʴ�Ϊ��BCAFED��
��A������Ͳ��ȡŨ����ʱ���ӹ۲찼Һ�棬�ᵼ����ȡҺ������ƫС��������ҺŨ��ƫС����A��ѡ��
B����Һע������ƿǰû�лָ������¾ͽ��ж��ݣ�����Һ�����ƫС����ҺŨ��ƫ��Bѡ��
C������ʱ���ӿ̶��ߣ�����Һ�����ƫС����ҺŨ��ƫ��Cѡ��
D��������ǰ����֪Ũ�ȵ�ϡ������ϴ����ƿ���ᵼ�����ʵ����ʵ���ƫ����ҺŨ��ƫ��Dѡ��
�ʴ�Ϊ��BCD��
��4��n��HCl��=
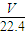 mol��m��HCl��=
mol��m��HCl��=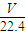 mol×36.5g/mol=
mol×36.5g/mol=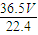 g��
g��m����Һ��=m��HCl��+m��ˮ��=
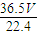 g+1000g��
g+1000g��V����Һ��=
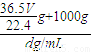 =
=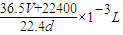 ��
��c��HCl��=
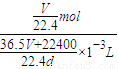 =
= mol/L��
mol/L���ʴ�Ϊ��
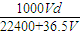 ��
����5��n��Cl-��=0.1L×0.5mol/L+2×0.2L×0.1mol/L=0.09mol��
V=100mL+200ml=300ml=0.3L��
c��Cl-��=
 =0.3mol/L��
=0.3mol/L���ʴ�Ϊ��0.3mol/L��
���������⿼���Ϊ�ۺϣ������Һ�����ơ�ʹ���������������衢�������Լ���ҺŨ�ȵļ��㣬��Ŀ�Ѷ��еȣ����ñ��⣬һ����Ҫ�к�ʵ�Ļ���֪ʶ�����ǰ������ʵ���Ũ�ȵļ��㷽����

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
 ��ͼΪʵ����ijŨ�����Լ�ƿ�ϵı�ǩ���Ը����й����ݻش��������⣺
��ͼΪʵ����ijŨ�����Լ�ƿ�ϵı�ǩ���Ը����й����ݻش��������⣺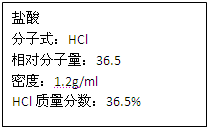 ��ͼΪʵ����ijŨ�����Լ�ƿ�ϵı�ǩ���Ը����й����ݻش��������⣺
��ͼΪʵ����ijŨ�����Լ�ƿ�ϵı�ǩ���Ը����й����ݻش��������⣺
 ��ͼΪʵ����ijŨ�����Լ�ƿ�ı�ǩ�ϵ��й����ݣ��Իش��������⣺
��ͼΪʵ����ijŨ�����Լ�ƿ�ı�ǩ�ϵ��й����ݣ��Իش��������⣺ ��ͼΪʵ����ijŨ�����Լ�ƿ�ϵı�ǩ���й����ݣ��Ը��ݱ�ǩ�ϵ��й����ݻش��������⣺
��ͼΪʵ����ijŨ�����Լ�ƿ�ϵı�ǩ���й����ݣ��Ը��ݱ�ǩ�ϵ��й����ݻش��������⣺