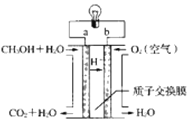
��Ŀ����
����Ŀ��ʵ��������ͼװ����ȡijЩ���岢����һϵ�е�����̽����
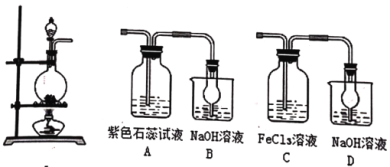
��ش�
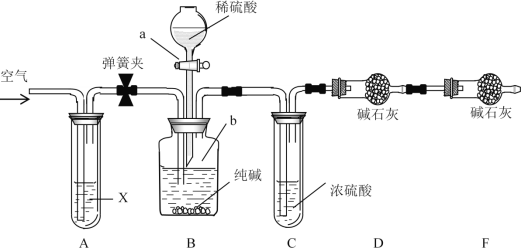
(1)����˵����ȷ����_____��
A.ʵ�鿪ʼʱ����װ�â��з�Һ©����������Һ����˳������
B.��װ�â�������ӣ���ȡCl2�����������ʣ�����Bװ�õ�����Ϊ������
C.������ͭ��Ũ������װ�â��з�Ӧ����Ӧ�����Һ�����ʽ�Ϊ����ͭ
D.��װ�â�͢����ӣ���ȡSO2�����������ʣ�A�е���ɫʯ����Һֻ�ܱ��
(2)��װ�â��װ�â����ӣ���ȡSO2�����������ʡ�C����Һ��ɫ���ػ�ɫ��dz��ɫ����Ӧ�����ӷ���ʽΪ��_____����Ӧ������C�е������ӵļ�ⷽ��Ϊ______��
���𰸡�D 2Fe3++SO2+2H2O=2Fe2++SO42-+4H+ ȡʣ����Һ���������Թ��У����Թ��еμ��Ȼ�����Һ����������ɫ����˵����Һ�к���SO42-��������Һ����SO42-
��������
(1)A.ʵ�鿪ʼʱ����Һ©����������ҲҪ������IJ�������
B.�����ж��������������Ҫ������������Һ���գ�
C.ͭ����ϡ���ᷴӦ����Ӧ����Һ��һ���������
D.��������Ϊ�������������������Ư�����ָʾ����
(2)������������뱵���ӽ�ϲ���������������ᱵ������
(1)A.ʵ�鿪ʼʱ��ֻ���Һ©������������Һ©���IJ����������������ѹ���ڷ�Һ©������ѹ��Һ�岻��˳�����£�A����
B.��װ�â�������ӣ���ȡCl2�����������ʣ����������ж�����Ҫ��Bװ�����գ����������Ⱦ��B����
C.������ͭ��Ũ������װ�â����ڼ��������·�Ӧ�����ŷ�Ӧ�Ľ��У�Ũ������ϡ���������ϡ������ͭ���ܷ�����Ӧ����Ӧֹͣ����˷�Ӧ�����Һ������Ϊ���������ͭ��C����
D.��װ�â�͢����ӣ���ȡSO2�����������ʣ�����������Һ�����ԣ���A�е���ɫʯ����Һ��죬���ڶ���������Ư�����ָʾ������A����ɫʯ����Һֻ�ܱ�죬D��ȷ��
�ʺ���ѡ����D��
(2)C����Һ��ɫ���ػ�ɫ��dz��ɫ��˵��Fe3+��SO2��Ӧ����Fe2+���÷�Ӧ�����ӷ���ʽΪ��2Fe3++SO2+2H2O=2Fe2++SO42-+4H+����Ӧ��C�����ɵ�SO42-����Ba2+��ϲ������������BaSO4��������������Ϊ��ȡʣ����Һ���������Թ��У����Թ��еμ��Ȼ�����Һ����������ɫ����˵����Һ�к���SO42-��������Һ����SO42-��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�