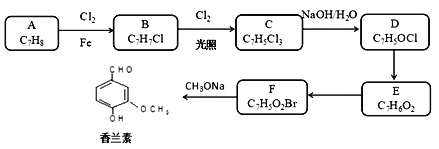
��Ŀ����
����Ŀ��W��X��Y��Z��ԭ���������������ͬһ������Ԫ�أ�W��X�ǽ���Ԫ�أ�Y��Z�Ƿǽ���Ԫ�أ�W��O2��Ӧ���������������
(1)д��W��O2��Ӧ�Ļ�ѧ����ʽ��_________��_________��
(2)W��Y���γɻ�����W2Y���û�����Ļ�ѧʽΪ____��X��Z���γɻ�����XZ2���û����������Ϊ____��
(3)�Ƚ�Y��Z��̬�⻯����ȶ��ԣ�____>____(�û�ѧʽ��ʾ)��
(4)W��X��Y��Z����Ԫ�ؼ����ӵ����Ӱ뾶�ɴ�С��˳����________(�û�ѧʽ��ʾ)��
���𰸡�4Na+O2![]() 2Na2O 2Na+O2
2Na2O 2Na+O2![]() Na2O2 Na2S �Ȼ�þ HCl H2S S2->Cl->Na+>Mg2+
Na2O2 Na2S �Ȼ�þ HCl H2S S2->Cl->Na+>Mg2+
��������
W��X��Y��Z��ԭ���������������ͬһ������Ԫ�أ�ԭ��������С��18��W��O2��Ӧ���������������W�ǽ���Ԫ�أ���WΪNaԪ�أ��γɵ��������������ƺ������ƣ�X�ǽ���Ԫ�أ���XΪMg��Al��Y��Z�Ƿǽ���Ԫ�أ���Si��P��S��Cl�е����֣����(2)�л�ѧʽ����ʾ�������
(1)����O2��Ӧ�Ļ�ѧ����ʽΪ��4Na + O2 =2Na2O��2Na + O2 ![]() Na2O2���ʴ�Ϊ��4Na + O2 =2Na2O��2Na + O2
Na2O2���ʴ�Ϊ��4Na + O2 =2Na2O��2Na + O2 ![]() Na2O2��
Na2O2��
(2)W(Na)��Y���γɻ�����W2Y��Y����-2�ۣ���YΪS���γɵĻ�����ΪNa2S����ZΪCl��X��Z���γɻ�����XZ2����XΪMg���γɵĻ�����Ϊ�Ȼ�þ���ʴ�Ϊ��Na2S���Ȼ�þ��
(3)ͬһ���ڴ������ң��ǽ�������ǿ����Ӧ�⻯����ȶ�����ǿ���ǽ�����Y��Z������(2)�ķ�������̬�⻯����ȶ��ԣ�HCl��H2S���ʴ�Ϊ��HCl��H2S��
(4)���Ӳ�ṹ��ͬ�����ӣ��˵����Խ�����Ӱ뾶ԽС�����Ӳ�Խ�࣬���Ӱ뾶Խ�����Ӱ뾶��С˳���ǣ�S 2-��Cl-��Na+��Mg2+���ʴ�Ϊ��S 2-��Cl-��Na+��Mg2+��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�