ћвƒњƒЏ»Ё
°Њћвƒњ°њ іњћЌ≠÷∆ѕя¬Ј∞еµƒ іњћ“Ї÷÷јаЇ№ґа£ЇЋб–‘ іњћ“Ї£ђ»з![]() іњћ»№“Ї°ҐH2O2-—ќЋбЉ∞CuCl2-—ќЋб
іњћ»№“Ї°ҐH2O2-—ќЋбЉ∞CuCl2-—ќЋб![]() Љо–‘ іњћ“Ї£ђ»з
Љо–‘ іњћ“Ї£ђ»з![]() »№“Їµ»°£їЎірѕ¬Ѕ–ќ ћв£Ї
»№“Їµ»°£їЎірѕ¬Ѕ–ќ ћв£Ї
£®1£©–і≥цѕ¬Ѕ– іњћ“Ї іњћЌ≠µƒјл„”Јљ≥ћ љ°£
ҐўFeCl3»№“Ї£Ї_______°£
ҐЏH2O2-—ќЋб£Ї_______°£
Ґџ![]() »№“Ї£Ї_______
»№“Ї£Ї_______![]() …ъ≥…
…ъ≥…![]() °£
°£
£®2£©јы”√Јѕ![]() іњћ“Ї
іњћ“Ї![]() Їђ
Їђ![]() °Ґ
°Ґ![]() Љ∞
Љ∞![]() ÷∆±ЄЉо–‘ іњћ“Ї
÷∆±ЄЉо–‘ іњћ“Ї![]() »№“ЇЇЌ
»№“ЇЇЌ![]() µƒ÷ч“™≤љ÷и∞ьј®£Ї”√
µƒ÷ч“™≤љ÷и∞ьј®£Ї”√![]() —хїѓЈѕ іњћ“Ї£ђЌ®»л∞±∆ш
—хїѓЈѕ іњћ“Ї£ђЌ®»л∞±∆ш![]() £ђєћ“ЇЈ÷јл£ђ”√—ќЋб»№љв≥ЅµнЇЌ
£ђєћ“ЇЈ÷јл£ђ”√—ќЋб»№љв≥ЅµнЇЌ![]() µƒ÷∆±Є°£ѕ¬Ѕ– µ—й„∞÷√≤їƒ№іпµљ µ—йƒњµƒµƒ «_______
µƒ÷∆±Є°£ѕ¬Ѕ– µ—й„∞÷√≤їƒ№іпµљ µ—йƒњµƒµƒ «_______![]() ћо„÷ƒЄ
ћо„÷ƒЄ![]() °£
°£
A. 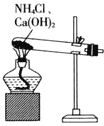 ÷∆±Є
÷∆±Є![]()
B. 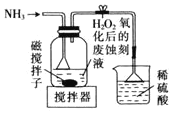 ÷∆±Є
÷∆±Є![]() ≤Ґ≥Ѕћъ
≤Ґ≥Ѕћъ
C. 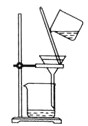 Ј÷јл
Ј÷јл![]() »№“ЇЇЌ
»№“ЇЇЌ![]() ≥Ѕµн
≥Ѕµн
D. 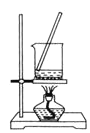 љЂ
љЂ![]() »№“Ї’фЄ…÷∆±Є
»№“Ї’фЄ…÷∆±Є![]()
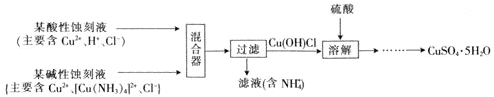
£®3£©≥£”√ЋЃЇѕл¬![]() їє‘≠Ј®їЎ ’CuCl2-—ќЋб іњћ“Їµ√µљµ•÷ Ќ≠£ђѕ»”√«в—хїѓƒ∆»№“Ї÷–ЇЌ∆д÷–Ћб≤ҐљЂ»№“Їµч÷Ѕ«њЉо–‘£ђ»їЇуЉ”»лЋЃЇѕл¬µ√ƒ…√„Ќ≠£ђЄ√—хїѓїє‘≠Јі”¶÷–—хїѓЉЅ”лїє‘≠ЉЅµƒќп÷ µƒЅњ÷Ѓ±»ќ™_______°£
їє‘≠Ј®їЎ ’CuCl2-—ќЋб іњћ“Їµ√µљµ•÷ Ќ≠£ђѕ»”√«в—хїѓƒ∆»№“Ї÷–ЇЌ∆д÷–Ћб≤ҐљЂ»№“Їµч÷Ѕ«њЉо–‘£ђ»їЇуЉ”»лЋЃЇѕл¬µ√ƒ…√„Ќ≠£ђЄ√—хїѓїє‘≠Јі”¶÷–—хїѓЉЅ”лїє‘≠ЉЅµƒќп÷ µƒЅњ÷Ѓ±»ќ™_______°£
£®4£©ќƒѕ„±®µј“ї÷÷їЎ ’ іњћЈѕ“Ї÷–Ќ≠≤Ґ÷∆»°µ®Јѓ![]() µƒ÷ч“™є§“’Ѕч≥ћ»зѕ¬£Ї
µƒ÷ч“™є§“’Ѕч≥ћ»зѕ¬£Ї
“—÷™£Ї![]() “„”лЋбЈі”¶°£
“„”лЋбЈі”¶°£
![]() ‘ЏїмЇѕ∆ч÷–£ђ
‘ЏїмЇѕ∆ч÷–£ђ![]() µƒјл„”Јљ≥ћ љќ™_______°£
µƒјл„”Јљ≥ћ љќ™_______°£
![]() »№”ЏЅтЋбµƒјл„”Јљ≥ћ љќ™_______°£
»№”ЏЅтЋбµƒјл„”Јљ≥ћ љќ™_______°£
°Њір∞Є°њ![]()
![]()
![]()
![]()
![]()
![]()
![]()
°Њљвќц°њ
£®1£©Ґў¬»їѓћъ»№“Ї”лЌ≠µ•÷ Јі”¶…ъ≥…¬»їѓ—«ћъЇЌ¬»їѓЌ≠£ђјл„”Јљ≥ћ љќ™£Ї![]() £ђє ір∞Єќ™£Ї
£ђє ір∞Єќ™£Ї![]() £ї
£ї
![]() єэ—хїѓ«в°Ґ—ќЋб”лЌ≠ЈҐ…ъ—хїѓїє‘≠Јі”¶…ъ≥…ЋЃЇЌ¬»їѓЌ≠£ђґ‘”¶µƒјл„”Јљ≥ћ љќ™£Ї
єэ—хїѓ«в°Ґ—ќЋб”лЌ≠ЈҐ…ъ—хїѓїє‘≠Јі”¶…ъ≥…ЋЃЇЌ¬»їѓЌ≠£ђґ‘”¶µƒјл„”Јљ≥ћ љќ™£Ї![]() £ђє ір∞Єќ™£Ї
£ђє ір∞Єќ™£Ї![]() £ї
£ї
![]() »№“Ї”лЌ≠µ•÷ Јі”¶…ъ≥…—«Ќ≠јл„”µƒ¬зЇѕќп£ђјл„”Јљ≥ћ љќ™£Ї
»№“Ї”лЌ≠µ•÷ Јі”¶…ъ≥…—«Ќ≠јл„”µƒ¬зЇѕќп£ђјл„”Јљ≥ћ љќ™£Ї![]() £ђє ір∞Єќ™£Ї
£ђє ір∞Єќ™£Ї![]() £ї
£ї
£®2£©A.Љ„„∞÷√њ…”√”Џ µ—й “÷∆»°∞±∆ш£ђє A≤ї—°£ї
B.”√„∞÷√““÷∆±Є![]() ≤Ґ≥Ѕћъ£ђєэ—хїѓ«вњ…љЂµЌЉџћъ—хїѓќ™ћъјл„”£ђі”ґшЌ®єэµчљЏpH≥э»•ћъ£ђє B≤ї—°£ї
≤Ґ≥Ѕћъ£ђєэ—хїѓ«вњ…љЂµЌЉџћъ—хїѓќ™ћъјл„”£ђі”ґшЌ®єэµчљЏpH≥э»•ћъ£ђє B≤ї—°£ї
C.„∞÷√±ыќ™єэ¬Ћ„∞÷√£ђ“тіЋњ…“‘Ј÷јл![]() »№“ЇЇЌ
»№“ЇЇЌ![]() ≥Ѕµн£ђє C≤ї—°£ї
≥Ѕµн£ђє C≤ї—°£ї
D.¬»їѓћъ‘ЏЋЃ»№“Ї÷–ƒ№єїЈҐ…ъЋЃљвЈі”¶£ђ’фЄ…µ√≤їµљ![]() £ђє D—°£ї
£ђє D—°£ї
є —°D£ї
£®3£©≥£”√ЋЃЇѕл¬![]() їє‘≠Ј®їЎ ’CuCl2-—ќЋб іњћ“Їµ√µљµ•÷ Ќ≠£ђЄщЊЁµ√ Іµз„” ЎЇгЈ÷ќцњ…÷™Є√—хїѓїє‘≠Јі”¶÷–—хїѓЉЅ”лїє‘≠ЉЅµƒќп÷ µƒЅњ÷Ѓ±»ќ™
їє‘≠Ј®їЎ ’CuCl2-—ќЋб іњћ“Їµ√µљµ•÷ Ќ≠£ђЄщЊЁµ√ Іµз„” ЎЇгЈ÷ќцњ…÷™Є√—хїѓїє‘≠Јі”¶÷–—хїѓЉЅ”лїє‘≠ЉЅµƒќп÷ µƒЅњ÷Ѓ±»ќ™![]() £ђє ір∞Єќ™£Ї
£ђє ір∞Єќ™£Ї![]() £ї
£ї
£®4£©‘ЏїмЇѕ∆ч÷–£ђ![]() µƒјл„”Јљ≥ћ љќ™
µƒјл„”Јљ≥ћ љќ™![]() £ђє ір∞Єќ™£Ї
£ђє ір∞Єќ™£Ї![]() £ї
£ї
![]() »№”ЏЅтЋбµƒјл„”Јљ≥ћ љќ™
»№”ЏЅтЋбµƒјл„”Јљ≥ћ љќ™![]() £ђє ір∞Єќ™£Ї
£ђє ір∞Єќ™£Ї![]() °£
°£

°Њћвƒњ°њҐс£Ѓѕ¬±н «ƒ≥ ≥∆Ј∞ь„∞іь…ѕµƒЋµ√ч£ђі”±н÷–µƒ≈дЅѕ÷–Ј÷±р—°≥ц“ї÷÷ќп÷ ‘Џѕа”¶µƒЇбѕя…ѕ°£
∆Ј√ы | °Ѕ°Ѕ°Ѕ |
≈дЅѕ | ѕ Љ¶µ∞°ҐЊЂ√жЈџ°Ґ∞„…∞ћ«°ҐЊЂЅґ÷≤ќп”Ќ°Ґƒћ”Ќ°ҐƒћЈџ°Ґ ≥—ќ°Ґƒы√ ÷≠µ» |
±£÷ ∆Џ | 240ћм |
…ъ≤ъ»’∆Џ | ±к”Џ∞ь„∞іьіьЈвњЏ…ѕ |
(1)ЄїЇђµ∞∞„÷ µƒќп÷ «___________________________£ї
(2)ЄїЇђћ«јаµƒќп÷ «____________________________£ї
(3)ЄїЇђ”Ќ÷ђµƒќп÷ «____________________________£ї
(4)ЄїЇђќђ…ъЋЎµƒќп «___________________________°£
Ґт.Є÷ћъ‘Џ≥± ™µƒњ’∆шјпЇ№њмЊЌ±їЄѓ і£ђ‘≠“т «‘ЏЄ÷ћъµƒ±н√ж–ќ≥…ЅЋ“ї≤гµзљв÷ »№“Їµƒ±°ƒ§£ђЄъЄ÷ћъјпµƒћъЇЌ…ўЅњµƒћЉєє≥…ќё эќҐ–°µƒ‘≠µз≥Ў£ђ«л–і≥цЅљЉЂЈҐ…ъµƒ÷ч“™µƒµзЉЂ љќ™£ЇЄЇЉЂ________________£ї’эЉЂ_____________°£
°Њћвƒњ°њ“—÷™£ЇҐў1molЊІћеєи÷–Їђ”–2molSi°™SiЉь°£
ҐЏSi(s)£ЂO2(g)===SiO2(g) ¶§H£ђ∆дЈі”¶єэ≥ћ”лƒ№Ѕњ±дїѓ»зЌЉЋщ Њ°£
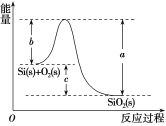
Ґџ
їѓ—ІЉь | Si°™O | O=O | Si°™Si |
ґѕњ™1 molє≤ЉџЉьЋщ–иƒ№Ѕњ/kJ | 460 | 500 | 176 |
ѕ¬Ѕ–ЋµЈ®÷–’э»Јµƒ «£® £©
A.ЊІћеєиєвЈьЈҐµз «љЂїѓ—Іƒ№„™їѓќ™µзƒ№B.ґю—хїѓєиќ»ґ®–‘–°”Џєиµƒќ»ґ®–‘
C.¶§H£љ£≠988kJ°§mol£≠1D.¶§H£љ(a£≠c)kJ°§mol£≠1