��Ŀ����
A��B��C��D��E��F��G��H��I��J ��Ϊ�л��������ת����ϵ��ͼ��ʾ����֪B �� C ��Ϊ��֧�����л������C�ڷ�Ӧ����ֻ������һ���л����G��ʹ������Ȼ�̼��Һ��ɫ���ش����⣺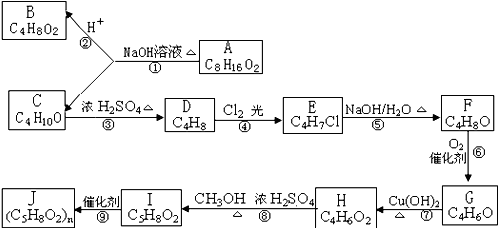
��1��G�Ľṹ��ʽ�� ���ߵķ�Ӧ���� ��
��2������ݵĻ�ѧ����ʽ ��
��3��A�ڸ������������¿ɷ�����ȥ��Ӧ�õ�D����д������ȥ��Ӧ�г�D�����һ������Ľṹ��ʽ ��
��4��������еġ�Cl2���⡱�ܷ��Ϊ��������ˮ������˵��ԭ��
��5��д����H ������ͬ�����ŵ�ͬ���칹��Ľṹ��ʽ
��6����һ�������£����봼�ᷢ���������������µ����ʹ����볢����дI��F��������������Ӧ����ʽ�� ��
��7��һ�������£�G��H����1��1�ۺϣ��ۺϲ����ж��֣�д���������־ۺϲ���Ľṹ��ʽ�� ��

��1��G�Ľṹ��ʽ��
��2������ݵĻ�ѧ����ʽ
��3��A�ڸ������������¿ɷ�����ȥ��Ӧ�õ�D����д������ȥ��Ӧ�г�D�����һ������Ľṹ��ʽ
��4��������еġ�Cl2���⡱�ܷ��Ϊ��������ˮ������˵��ԭ��
��5��д����H ������ͬ�����ŵ�ͬ���칹��Ľṹ��ʽ
��6����һ�������£����봼�ᷢ���������������µ����ʹ����볢����дI��F��������������Ӧ����ʽ��
��7��һ�������£�G��H����1��1�ۺϣ��ۺϲ����ж��֣�д���������־ۺϲ���Ľṹ��ʽ��
������A����������ˮ��Һ���ữ�õ�B��ͬʱ����C��C�ķ���ʽC4H10O��CΪ������֪AΪ����BΪ���ᣬB��C��Ϊ��֧�����л��������BΪCH3CH��CH3��COOH��C�ڷ�Ӧ����ֻ�������л�����D��D�ķ���ʽΪC4H8����DΪCH2=C��CH3��2��D��������������ȡ����Ӧ����E��E����������ˮ��Һ��ˮ������F��F�к���-OH��F����������G����F��G�ķ���ʽ��֪���ǻ�������Ϊȩ����G����-CHO��������C=C˫����G������������ͭ��Ӧ����H��H����-COOH����HΪCH2=C��CH3��COOH��GΪCH2=C��CH3��CHO��FΪCH2=C��CH3��CH2OH��EΪCH2=C��CH3��CH2Cl��H��״�����������Ӧ����I��IΪCH2=C��CH3��COOCH3��I�����Ӿ۷�Ӧ����J���ݴ˽��
����⣺A����������ˮ��Һ���ữ�õ�B��ͬʱ����C��C�ķ���ʽC4H10O��CΪ������֪AΪ����BΪ���ᣬB��C��Ϊ��֧�����л��������BΪCH3CH��CH3��COOH��C�ڷ�Ӧ����ֻ�������л�����D��D�ķ���ʽΪC4H8����DΪCH2=C��CH3��2��D��������������ȡ����Ӧ����E��E����������ˮ��Һ��ˮ������F��F�к���-OH��F����������G����F��G�ķ���ʽ��֪���ǻ�������Ϊȩ����G����-CHO��������C=C˫����G������������ͭ��Ӧ����H��H����-COOH����HΪCH2=C��CH3��COOH��GΪCH2=C��CH3��CHO��FΪCH2=C��CH3��CH2OH��EΪCH2=C��CH3��CH2Cl��H��״�����������Ӧ����I��IΪCH2=C��CH3��COOCH3��I�����Ӿ۷�Ӧ����J��
��1��������������֪��G�Ľṹ��ʽΪCH2=C��CH3��CHO��
��Ӧ����CH2=C��CH3��CHO����������CH2=C��CH3��COOH��
�ʴ�Ϊ��CH2=C��CH3��CHO��������Ӧ��
��2����Ӧ����CH2=C��CH3��CH2Cl����ˮ�ⷴӦ����CH2=C��CH3��CH2OH����Ӧ����ʽΪ��
CH2=C��CH3��CH2Cl+NaOH
CH2=C��CH3��CH2OH+NaCl��
�ʴ�Ϊ��CH2=C��CH3��CH2Cl+NaOH
CH2=C��CH3��CH2OH+NaCl��
��3��A�ڸ������������¿ɷ�����ȥ��Ӧ�õ�C4H8���ɷ���ʽ��֪���A�Ľṹ��֪������ȥ��Ӧ�������һ������Ľṹ��ʽΪCH3CH��CH3��COOH���ʴ�Ϊ��CH3CH��CH3��COOH��
��4�������á�������ˮ����ᷢ���ӳɷ�Ӧ���ʲ��У�
�ʴ�Ϊ�����У������á�������ˮ����ᷢ���ӳɷ�Ӧ��
��5��HΪCH2=C��CH3��COOH����H������ͬ�����ŵ�ͬ���칹��Ľṹ��ʽΪCH2=CHCH2COOH��CH3CH=CHCOOH��
�ʴ�Ϊ��CH2=CHCH2COOH��CH3CH=CHCOOH��
��6����һ�������£����봼�ᷢ���������������µ����ʹ���CH2=C��CH3��COOCH3��CH2=C��CH3��CH2OH��������������Ӧ����ʽ��CH2=C��CH3��COOCH3+CH2=C��CH3��CH2OH
CH2=C��CH3��COOCH2��CH3��C=CH2+CH3OH��
�ʴ�Ϊ��CH2=C��CH3��COOCH3+CH2=C��CH3��CH2OH
CH2=C��CH3��COOCH2��CH3��C=CH2+CH3OH��
��7��һ�������£�CH2=C��CH3��CHO��CH2=C��CH3��COOH����1��1�ۺϣ��ۺϲ����ж��֣��ۺϲ���Ľṹ��ʽ��
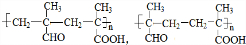 �ȣ�
�ȣ�
�ʴ�Ϊ��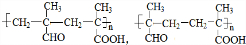 ��
��
��1��������������֪��G�Ľṹ��ʽΪCH2=C��CH3��CHO��
��Ӧ����CH2=C��CH3��CHO����������CH2=C��CH3��COOH��
�ʴ�Ϊ��CH2=C��CH3��CHO��������Ӧ��
��2����Ӧ����CH2=C��CH3��CH2Cl����ˮ�ⷴӦ����CH2=C��CH3��CH2OH����Ӧ����ʽΪ��
CH2=C��CH3��CH2Cl+NaOH
| ˮ |
| �� |
�ʴ�Ϊ��CH2=C��CH3��CH2Cl+NaOH
| ˮ |
| �� |
��3��A�ڸ������������¿ɷ�����ȥ��Ӧ�õ�C4H8���ɷ���ʽ��֪���A�Ľṹ��֪������ȥ��Ӧ�������һ������Ľṹ��ʽΪCH3CH��CH3��COOH���ʴ�Ϊ��CH3CH��CH3��COOH��
��4�������á�������ˮ����ᷢ���ӳɷ�Ӧ���ʲ��У�
�ʴ�Ϊ�����У������á�������ˮ����ᷢ���ӳɷ�Ӧ��
��5��HΪCH2=C��CH3��COOH����H������ͬ�����ŵ�ͬ���칹��Ľṹ��ʽΪCH2=CHCH2COOH��CH3CH=CHCOOH��
�ʴ�Ϊ��CH2=CHCH2COOH��CH3CH=CHCOOH��
��6����һ�������£����봼�ᷢ���������������µ����ʹ���CH2=C��CH3��COOCH3��CH2=C��CH3��CH2OH��������������Ӧ����ʽ��CH2=C��CH3��COOCH3+CH2=C��CH3��CH2OH
| һ������ |
�ʴ�Ϊ��CH2=C��CH3��COOCH3+CH2=C��CH3��CH2OH
| һ������ |
��7��һ�������£�CH2=C��CH3��CHO��CH2=C��CH3��COOH����1��1�ۺϣ��ۺϲ����ж��֣��ۺϲ���Ľṹ��ʽ��
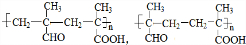 �ȣ�
�ȣ��ʴ�Ϊ��
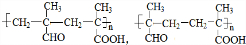 ��
�����������⿼���л�����ƶϣ��漰��������ȩ�����ᡢϩ��������ת���ȣ��Ѷ��еȣ�������Ŀ��Ϣ����Ϸ�Ӧ���������ƶϣ������ͻ�ƿ���C��D�ķ���ʽ����Ϸ�Ӧ����������Ӧ��Ϣ���������������ϵķ����ƶϣ��Ѷ��еȣ�

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
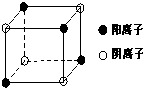 [��ѧ/ѡ��/���ʽṹ������]A��B��C��D��E���ֶ�����Ԫ�أ�ԭ��������������Ԫ�ض�Ӧ�ĵ��ʾ�Ϊ���壮A��C��E��Ԫ�ص�ԭ�Ӻ����ֻ��2��δ�ɶԵ��ӣ�B��EԪ�ص�ԭ������֮�͵���C��DԪ�ص�ԭ������֮�ͣ�
[��ѧ/ѡ��/���ʽṹ������]A��B��C��D��E���ֶ�����Ԫ�أ�ԭ��������������Ԫ�ض�Ӧ�ĵ��ʾ�Ϊ���壮A��C��E��Ԫ�ص�ԭ�Ӻ����ֻ��2��δ�ɶԵ��ӣ�B��EԪ�ص�ԭ������֮�͵���C��DԪ�ص�ԭ������֮�ͣ�