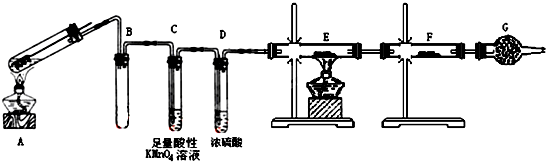
��Ŀ����
8��������ѧij��ѧ��ȤС��Ϊ��̽������ϩ�Ļ�ѧ���ʡ���������������ʵ�飺������ѧ֪ʶ�ش��������⣮��1������ϩ����ͨ�����CCl4��Һ�У�ʵ����������Һ��ɫ��ȥ��������Ӧ�Ļ�ѧ����ʽΪ��CH2=CH2+Br2��CH2Br-CH2Br���÷�Ӧ������Ϊ�ӳɷ�Ӧ��
��2������ϩ����ͨ�뵽���Ը��������Һ�У�ʵ�������Ǹ��������Һ��ɫ��ȥ��������������Ը����������������ϩ����
��3����ϩ��ˮ��һ�������µĻ�ѧ��Ӧ����ʽΪ��CH2=CH2+H2O$\stackrel{һ������}{��}$CH3CH2OH��
��4������Ϊ��ϩ��ͬϵ��ڱ�״����5.6L������Ϊ10.5g��10.5g������ȫȼ��ʱ����16.8L������£�������̼��13.5gˮ������������Ӿ۷�Ӧ�Ļ�ѧ����ʽ��
 ��
��
���� ��1����ϩ����̼̼˫���������巢���ӳɷ�Ӧ����CH2Br-CH2Br����Һ��ɫ��
��2����ϩ�ɱ����Ը��������Һ������������ر���ԭ��
��3����ϩ��ˮ�����ӳɷ�Ӧ�����Ҵ���
��4��n=$\frac{5.6L}{22.4L/mol}$=0.25mol��M=$\frac{10.5g}{0.25mol}$=42g/mol��n��CO2��=$\frac{16.8L}{22.4L/mol}$=0.75mol����֪����3��Cԭ�ӣ�n��$\frac{13.5g}{18g/mol}$��=0.75mol����֪�л��ﺬ��6��Hԭ�ӣ�
��� �⣺��1����ϩ����̼̼˫���������巢���ӳɷ�Ӧ����CH2Br-CH2Br������ʽΪ CH2=CH2+Br2��CH2Br-CH2Br����Һ��ɫ��
�ʴ�Ϊ����Һ��ɫ��ȥ�� CH2=CH2+Br2��CH2Br-CH2Br�� �ӳɷ�Ӧ��
��2����ϩ�ɱ����Ը��������Һ������������ر���ԭ���ɹ۲쵽���������Һ��ɫ��ȥ���ʴ�Ϊ�����������Һ��ɫ��ȥ��
��3����ϩ��ˮ�����ӳɷ�Ӧ�����Ҵ�������ʽΪCH2=CH2+H2O$\stackrel{һ������}{��}$CH3CH2OH���ʴ�Ϊ��CH2=CH2+H2O$\stackrel{һ������}{��}$CH3CH2OH��
��4��n=$\frac{5.6L}{22.4L/mol}$=0.25mol��M=$\frac{10.5g}{0.25mol}$=42g/mol��n��CO2��=$\frac{16.8L}{22.4L/mol}$=0.75mol����֪����3��Cԭ�ӣ�n��$\frac{13.5g}{18g/mol}$��=0.75mol����֪�л��ﺬ��6��Hԭ�ӣ���ӦΪ��ϩ�������Ӿ۷�Ӧ���ɾ۱�ϩ������ʽΪ ��
��
�ʴ�Ϊ�� ��
��
���� �����ۺϿ�����ϩ�����ʣ�Ϊ��Ƶ���㣬����ѧ���ķ��������������Ŀ��飬����ע������л�������ŵ������Լ�����ʽ���ƶ�˼·������ʱע����գ��ѶȲ���

��֪���ƻ�1mol��ѧ����Ҫ���յ��������±���ʾ��
| ��ѧ�� | C-H | O-H | C=O | H-H |
| ����������kJ/mol�� | 414 | 464 | 803 | 436 |
| A�� |  | B�� |  | C�� |  | D�� |  |
| A�� | ������ɾ�����ͨʽCnH2n+2 | |
| B�� | �����������ˮ | |
| C�� | ���������к�̼����� | |
| D�� | ����ֻ�ܷ���ȡ����Ӧ�����ܷ����ӳɷ�Ӧ |
| A�� | ��������ˮ�ε���ɫʯ����ֽ�ϣ���ֽ�ȱ�����ɫ��֤����ˮ����Ư���� | |
| B�� | ��HClO��Һ��ͨ��SO2������H2SO4��֤��H2SO4�����Ա�HClOǿ | |
| C�� | �������ھƾ��ƻ����ϼ��ȣ������ۻ��������䣬֤�����������۵������ | |
| D�� | ��Na2SiO3��Һ�еμӷ�̪����Һ��죬֤��Na2SiO3�����˷�Ӧ���ɼ��� |
| A�� | K+����Na+����OH-����SO42- | B�� | Mg2+����SO42-����NH4+����Cl- | ||
| C�� | K+����Na+����HCO3-����Cl- | D�� | K+���� Na+���� NO3-����CO32- |
| A�� | 17g����������ԭ����ΪNA | |
| B�� | ��״���£�1 Lˮ������������Ϊ$\frac{1}{22.4}$NA | |
| C�� | 0.3 mol/L��MgCl2��Һ�к�Mg2+��Ŀ0.3 NA | |
| D�� | ���³�ѹ�£�16 g O2���е���ԭ����ΪNA |
| A�� | ��״���£�2.24 L���к��еķ�����Ϊ0.1 NA | |
| B�� | ��״���£�2.24 L Cl2�����ϡNaOH��Һ��Ӧ��ת�Ƶĵ�������Ϊ0.1 NA | |
| C�� | 0.1 mol•L-1������ͭ��Һ�к�ͭ������Ϊ0.1 NA | |
| D�� | 1mol FeCl3��ˮ��ȫ��Ӧת��Ϊ����������������н������ӵ���ĿΪNA |
| A�� | �¶����ߣ�Ksp��AgCl������ | |
| B�� | ��AgClˮ��Һ�м���NaCl��Һ��Ksp��AgCl����� | |
| C�� | �����£���0.001 mol•L-1 AgNO3��Һ����0.001 mol•L-1 KCl�� 0.001 mol•L-1 K2CrO4��Һ�Ȳ���Ag2CrO4���� | |
| D�� | ��Mg2+Ϊ0.12 mol•L-1����Һ��Ҫ����Mg��OH��2������Ӧ������Һ��pH��9 |