��Ŀ����
����A��B��C��D��E��Fԭ�������������������Ԫ�أ�����λ��Ԫ�����ڱ���ǰ�����ڡ�BԪ�غ���3���ܼ�����ÿ���ܼ������ĵ�������ͬ��D��ԭ�Ӻ�����8���˶�״̬��ͬ�ĵ��ӣ�EԪ����FԪ�ش���ͬһ�������ڵ��壬���ǵ�ԭ���������3����EԪ�صĻ�̬ԭ����4��δ�ɶԵ��ӡ���ش��������⣺
��1����Ԫ�ط��ű�ʾB��C��D����Ԫ�صĵ�һ�������ɵ͵��ߵ�˳�� ��
��2������˵��������� ��
A�������������Է��������ȶ�����̼�����Էе㣺SiO2��CO2
B���縺��˳��C��N��O��F
C��N2��COΪ�ȵ����壬��ѧ��������
D������ˮ���Ӽ��������������ȶ��ԣ�H2O��H2S
��3��EԪ�ص�+2����������۵��+3�������� ����ͣ���ԭ���� ��
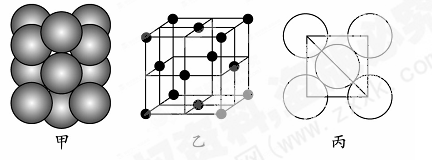
��4��B���ʵ�һ��ͬ��������ľ�����ͼ����ʾ����������ܶ�Ϊ��g/cm3�������ӵ�������ֵΪNA�����������������ԭ��֮��ľ���Ϊ cm��
��5��F�����������ڶ���ø�ĸ����ӣ��˹�ģ��ø�ǵ�ǰ�о����ȵ㡣
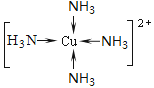
�� Fԭ�ӵ���Χ�����Ų�ʽΪ________����F����������Һ��ͨ�����C��A�γɵ�����N��������[F(N)4]2+�������ӵĽṹʽΪ ��
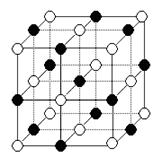
��ij��������F(��)(���ʾ���ϼ�Ϊ��1)����γ�ͼ����ʾ�����ӣ���������̼ԭ�ӵ��ӻ���ʽ�� ��


ͼ�� ͼ��
��1����Ԫ�ط��ű�ʾB��C��D����Ԫ�صĵ�һ�������ɵ͵��ߵ�˳�� ��
��2������˵��������� ��
A�������������Է��������ȶ�����̼�����Էе㣺SiO2��CO2
B���縺��˳��C��N��O��F
C��N2��COΪ�ȵ����壬��ѧ��������
D������ˮ���Ӽ��������������ȶ��ԣ�H2O��H2S
��3��EԪ�ص�+2����������۵��+3�������� ����ͣ���ԭ���� ��
��4��B���ʵ�һ��ͬ��������ľ�����ͼ����ʾ����������ܶ�Ϊ��g/cm3�������ӵ�������ֵΪNA�����������������ԭ��֮��ľ���Ϊ cm��
��5��F�����������ڶ���ø�ĸ����ӣ��˹�ģ��ø�ǵ�ǰ�о����ȵ㡣
�� Fԭ�ӵ���Χ�����Ų�ʽΪ________����F����������Һ��ͨ�����C��A�γɵ�����N��������[F(N)4]2+�������ӵĽṹʽΪ ��
��ij��������F(��)(���ʾ���ϼ�Ϊ��1)����γ�ͼ����ʾ�����ӣ���������̼ԭ�ӵ��ӻ���ʽ�� ��


ͼ�� ͼ��
��1��C<O<N(2��)
(2)ACD(2��)
(3)��(1��) Fe2+�뾶��Fe3+���������ҲС��Fe3+��FeO�ľ����ܱ�Fe2O3��С(2��)
��4��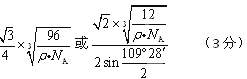
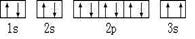
��5����3d104s1 ��1�֣� ��2�֣�
��sp2��sp3��2�֣�
(2)ACD(2��)
(3)��(1��) Fe2+�뾶��Fe3+���������ҲС��Fe3+��FeO�ľ����ܱ�Fe2O3��С(2��)
��4��
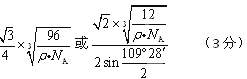

��5����3d104s1 ��1�֣� ��2�֣�
��sp2��sp3��2�֣�
���������BԪ�غ���3���ܼ�1s��2s��2p����ÿ���ܼ������ĵ�������ͬ������2�����ӣ���BΪCԪ�أ�D��ԭ�Ӻ�����8���˶�״̬��ͬ�ĵ��ӣ�����D��OԪ�أ���C��NԪ�أ�EԪ����FԪ�ش���ͬһ�������ڵ��壬���ǵ�ԭ���������3��˵��E��F�ǵ�������Ԫ�أ���Ϊǰ��������������Ԫ�ص�ԭ������ֻ��1��EԪ�صĻ�̬ԭ����4��δ�ɶԵ��ӣ���EΪ26��Ԫ��Fe��FΪ29��Ԫ��Cu��
��1��ͬ����Ԫ�ص�һ��������˵������������ߣ���N��2p��������ǰ�������Ƚ��ȶ�������ʧȥ������C��N��O����Ԫ�صĵ�һ�������ɵ͵��ߵ�˳����C<O<N��
��2��A������������ԭ�Ӿ��壬������̼�Ƿ��Ӿ��壬���Էе㣺SiO2��CO2��A����B��ͬ����Ԫ����˵�����������ǿ����ȷ��C��N2��COΪ�ȵ����壬�������ƵĻ�ѧ�ṹ������D��������۷е�ߵ��йأ���ΪO��ԭ�Ӱ뾶С��S��ԭ�Ӱ뾶������H2O�����й��ۼ���H2Sǿ������ˮ���ȶ�������ѡACD��
��3��FeO�ķе��Fe2O3�ͣ�����ΪFe2+�뾶��Fe3+���������ҲС��Fe3+��FeO�ľ����ܱ�Fe2O3��С��Ե�ʣ�
��4���þ�������8��1/8+6��1/2+4=8��Cԭ�ӣ��辧�����ⳤ��acm,���="8��12" NA/a3, ���������������ԭ��������Խ���һ������˵�Cԭ�ӹ��ɵ��������������붥��ľ��룬������ѧ֪ʶ����⣬��Ϊ
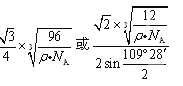 ��
����5����FΪ29��Cu����Χ���ӵ��Ų�ʽΪ3d104s1��NΪ������������ͭ��Һ��ͨ����������������İ���ͭ�����ӣ���ṹʽΪ

��C��N(��C)�Բ����ͼ����ʱ��sp2�ӻ����Ա��ͼ����ʱΪsp3�ӻ���

��ϰ��ϵ�д�
�����Ŀ
 ��Υ���� ԭ����
��Υ���� ԭ����