��Ŀ����
����Ŀ��CO2�ǵ�����ȡ֮������֮���ߵ�̼Դ����CO2Ӧ����������ʵ�����ۺ�������Ŀǰ���о��ȵ㡣
��1����CO2ת��Ϊ������CO2��Դ�����õ���Ҫ������
I.�ڴ���������CO2��CH4ת��ΪCH3COOH�ķ�Ӧ����ʾ��ͼ��ͼ��
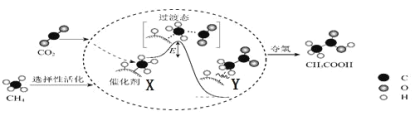
���ںϳ�CH3COOH�ķ�Ӧ�����У������й�˵����ȷ����_____��������ĸ��
a.�ô���ʹ��Ӧ��ƽ�ⳣ������
b.CH4��CH3COOH��������C��H����������
c.��X��Y�����зų��������γ���C��C��
�ڸ���������CO2��CH4�ϳ�CH3COOH�Ļ�ѧ����ʽΪ_____��
II.��ⷨת��CO2��HCOOH��ԭ����ͼ��

��д������CO2��ԭΪHCOO-�ĵ缫��Ӧʽ��_____��
�ڵ��һ��ʱ�����������KHCO3��ҺŨ�Ƚ��ͣ���ԭ����_____��
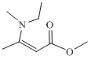
��2����CO2�ϳɼ״���CO2��Դ�����õ���Ҫ�������о������ڴ���������CO2��H2�ɷ�����Ӧ��CO2(g)+3H2(g)![]() CH3OH(g)+H2O(g) ��H
CH3OH(g)+H2O(g) ��H
����������ߺϳ�CH3OH��Ӧ��CO2��ƽ��ת���ʵĴ�ʩ��_____��������ĸ��
a.ʹ�ô��� b.��ѹ c.�����ʼͶ�ϱ�![]()
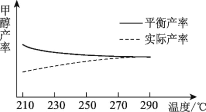
���о��¶ȶ��ڼ״����ʵ�Ӱ�졣��210��~290�汣��ԭ������CO2��H2��Ͷ�ϱȲ��䣬�õ��״���ʵ�ʲ��ʡ�ƽ��������¶ȵĹ�ϵ��ͼ��ʾ����H____0������������������������������____��
���𰸡�bc CH4+CO2![]() CH3COOH 2CO2+HCO3-+2e-=HCOO-+CO32- ��������O2��c��H+������c��HCO3-�����ͣ�K+���ֽ������� b < �¶����ߣ��״���ƽ����ʽ���
CH3COOH 2CO2+HCO3-+2e-=HCOO-+CO32- ��������O2��c��H+������c��HCO3-�����ͣ�K+���ֽ������� b < �¶����ߣ��״���ƽ����ʽ���
��������
(1)�����ٸ��ݺϳ�ʾ��ͼ�����жϣ�
����ͼʾд����CO2��CH4�ϳ�CH3COOH�Ļ�ѧ����ʽ��
�����ٸ���ͼʾ����������CO2�õ��ӣ�д��CO2��ԭΪHCOO-�ĵ缫��Ӧʽ��
�ڸ��ݵ��ط�Ӧԭ��������
(2)�ٸ���ƽ��ת����Ӱ�����ط��������
�ڸ���ͼ��ü״���ƽ����������¶ȵ����ߵı仯���ơ�
(1)������a������ֻ�ܸı仯ѧ��Ӧ���ʣ����ܸı䷴Ӧ��ƽ�ⳣ������a����
b����ͼ�б仯��֪�����ڴ���������ѡ���Ի�����������̼ԭ�ӻ�������γ��µĹ��ۼ�������C-H���������ѣ���b��ȷ��
c��X��Y����ֵ���ͣ�˵��Ϊ���ȹ��̣���CH4��CH3COOH��C-C���γɣ���c��ȷ��
�ʴ�ѡbc��
����ͼʾ��֪����CO2��CH4�ϳ�CH3COOH�Ļ�ѧ����ʽΪCH4+CO2![]() CH3COOH��
CH3COOH��
�����������õ��ӣ�CO2��ԭΪHCOO-�ĵ缫��ӦʽΪ��2CO2+HCO3-+2e-=HCOO-+CO32-��
������H2Oʧ���Ӳ���O2��c(H+)����̼����������������ӷ�Ӧ������c(HCO3-)���ͣ�K+���ֽ���������������������KHCO3��ҺŨ�Ƚ��ͣ�
(2)��a��ʹ�ô���ֻ�ܸı仯ѧ��Ӧ���ʣ���Ӱ�컯ѧƽ�⣬Ҳ�Ͳ�Ӱ��CO2��ƽ��ת���ʣ�
b���÷�Ӧ������ӦΪ���������С�ķ�Ӧ����ѹ��ʹ��ѧƽ�������ƶ����ܹ����CO2��ƽ��ת���ʣ�
c������CO2��H2�ij�ʼͶ�ϱȣ�CO2��ƽ��ת���ʽ��ͣ�
�ʴ�ѡb��
�ڸ���ͼ���֪���״���ƽ����������¶ȵ����߶����ͣ���������ӦΪ���ȷ�Ӧ������H<0��

 ȫ�ܲ����ĩС״Ԫϵ�д�
ȫ�ܲ����ĩС״Ԫϵ�д� ��Ȥ����¹�֪��ϵ�д�
��Ȥ����¹�֪��ϵ�д�����Ŀ����֪ijЩ���ۼ��ļ��������ʾ���Իش��������⣺��
���ۼ� | ���� | ���ۼ� | ���� |
| 436 |
| 467 |
| 243 |
| 945 |
| 413 |
| 431 |
��1��![]() ���ļ���Ϊʲô��
���ļ���Ϊʲô��![]() ���ļ��ܴ�_______��
���ļ��ܴ�_______��
��2����֪![]() ��
��![]() ʱ��
ʱ��![]() �ķ��ӷֽ⣬��
�ķ��ӷֽ⣬��![]() ��
��![]() ʱ������ȫ�ֽ�Ϊ
ʱ������ȫ�ֽ�Ϊ![]() ��
��![]() ���Խ������е�ԭ��_______��
���Խ������е�ԭ��_______��
��3���Խ��͵������ڿ������ȶ����ڵ�ԭ��_______��