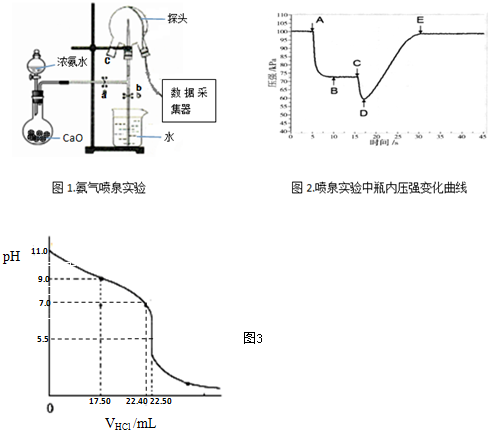
��Ŀ����
12������������Ԫ��X��Y��Z��W��ԭ��������������Xԭ�ӵ����������������ڲ��������2����Y�ǵؿ��к�����ߵ�Ԫ�أ�Z2+��Y2-������ͬ�ĵ��Ӳ�ṹ��W��Xͬ���壮����˵����ȷ���ǣ�������| A�� | ���Ӱ뾶��r��Y2-����r��Z2+�� | |
| B�� | Y�ֱ���Z��W�γɵĻ������л�ѧ��������ͬ | |
| C�� | Y����̬���⻯������ȶ��Աȵ�Ԫ�ص���̬���⻯���� | |
| D�� | X��Y��Z��W����Ԫ�ص�������۵������������������ |
���� Xԭ�ӵ����������������ڲ��������2�������X��C��Y�ǵؿ��к�����ߵ�Ԫ��O��W��Xͬ���壬W��Si��������Ԫ��Z2+��Y2-������ͬ�ĵ��Ӳ�ṹ�����ZΪMg��
A�������Ų���ͬ�����ӣ�ԭ������Խ�����Ӱ뾶ԽС��
B��MgO�к������Ӽ���SiO2�к��й��ۼ���
C��Ԫ�طǽ�����Խǿ������̬�⻯��Խ�ȶ���
D��Oû��������ۣ�
��� �⣺Xԭ�ӵ����������������ڲ��������2�������X��C��Y�ǵؿ��к�����ߵ�Ԫ��O��W��Xͬ���壬W��Si��������Ԫ��Z2+��Y2-������ͬ�ĵ��Ӳ�ṹ�����ZΪMg��
A��YΪO��ZΪMg�������Ų���ͬ�����ӣ�ԭ������Խ�����Ӱ뾶ԽС����r��Y2-����r��Z2+������A��ȷ��
B��Y�ֱ���Z��W�γɵĻ�����ֱ�ΪMgO��SiO2��MgO�к������Ӽ���SiO2�к��й��ۼ�����ѧ�����Ͳ�ͬ����B����
C���ǽ�����O��N��Ԫ�طǽ�����Խǿ������̬�⻯��Խ�ȶ������H2O�����ȶ��Դ���NH3����C����
D��Oû��������ۣ�C��Si��Mg��������۵����������������������D����
��ѡA��
���� ���⿼��ԭ�ӽṹ��Ԫ��������֪ʶ��������ѧ���ķ��������Ŀ��飬�Ѷ��еȣ�ע�����ԭ�ӽṹ�����Ͷ�Ӧ���ʡ�����������ʣ�ѧϰ��ע����ػ���֪ʶ�Ļ��ۣ�

| A�� | 4�� | B�� | 8�� | C�� | 12�� | D�� | 24�� |
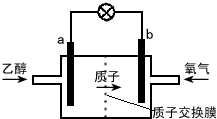
| A�� | a��Ϊ��صĸ������õ缫����������Ӧ | |
| B�� | ��ع���ʱ������a�������ص��߾����ݵ�b�� | |
| C�� | ��������ĵ缫��ӦʽΪ O2+2H2O+4e-�T4OH- | |
| D�� | ��ع���ʱ��1mol�Ҵ�������ת��12mol���� |
| A�� | pH=a�İ�ˮ��Һ��ϡ��10������pH=b����a=b+1 | |
| B�� | ����AgCl��AgI���������Һ��c��Ag+����c��Cl-��=c��I-�� | |
| C�� | 0.1mol/LNH4Cl��0.1mol/L��ˮ�������ϣ�pH��7����c��NH3•H2O����c��NH4+����c��Cl-����c��OH-�� | |
| D�� | �������ʵ�����NaHC2O4��Na2C2O4����Һ��2c��Na+��=3[c��HC2O4-��+c��C2O42-��+c��H2C2O4��] |
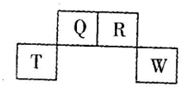
| A�� | ��̬�⻯���ȶ��ԣ�R��Q | |
| B�� | Ԫ��T�����ӽṹʾ��ͼΪ | |
| C�� | ��wͬ�����ijԪ���γɵ�18���ӵ��⻯�������ֻ�й��ۼ�û�����Ӽ� | |
| D�� | Q���γɶ��ֺ����� |
| A�� | 25 | B�� | 53 | C�� | 78 | D�� | 131 |
| A�� | NaOH | B�� | H2O | C�� | CaCl2 | D�� | H2SO4 |
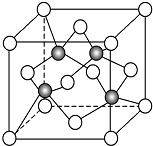 ̼���仯�����������ϵ����
̼���仯�����������ϵ���� ��C��N��O��Ԫ�صĵ�һ�������ɴ�С��˳��ΪN��O��C���ڱ�ϩ������̼ԭ�ӵ��ӻ���ʽΪsp2��sp3��
��C��N��O��Ԫ�صĵ�һ�������ɴ�С��˳��ΪN��O��C���ڱ�ϩ������̼ԭ�ӵ��ӻ���ʽΪsp2��sp3��