��Ŀ����
5����֪25��ʱ����������ĵ��볣�����£�| ����Ļ�ѧʽ | CH3COOH | HCN | H2S |
| ���볣�� | 1.8��10-4 | 4.9��10-11 | Ki=1.3��10-1 Kr=7.1��10-15 |
| A�� | �����ʵ���Ũ�ȵĸ���ҺpH��ϵΪpH��CH3COONa����pH��Na2S����pH��NaCN�� | |
| B�� | amol/LHCN��bmol/LNaOH��Һ�������ϣ�������Һ��c��Na+����c��CN-������aһ��С�ڻ����b | |
| C�� | NaHS��Na2S�Ļ����Һ�У�һ������c��Na+��+c��H+��=c��OH-��+c��HS-��+2c��S2-�� | |
| D�� | ijŨ�ȵ�NaCN��Һ��pH=d����������ˮ�������c��OH-��=10-dmol/L |
���� A������ƽ�ⳣ��Խ������Խǿ������̶�Խ��
B����Ũ��ʱ����NaCN��CN-���Ӳ���ˮ�⣬a�����Դ���b��
C�����ݻ��Һ�еĵ���غ������
D���ȼ�������������Ũ�ȣ�ˮ�������������Ũ�ȵ�������������Ũ�ȣ�
��� �⣺A���ɱ����е����ݿ�֪���������ƽ�ⳣ�������������ӵĵ���ƽ�ⳣ����С������Խǿ�����Ӧ�ε�ˮ��Խ���������ʵ���Ũ����Һ������������Ũ��ԽС�����Ũ�ȵ�������Һ��pH��ϵΪ��pH��Na2S����pH��NaCN����pH��CH3COONa������A����
B�������ʵ���ʱ����NaCN��CN-����ˮ�⣬��c��Na+����c��CN-��������a mol•L-1HCN��Һ��b mol•L-1NaOH��Һ�������Ϻ�������Һ��c��Na+����c��CN-����a�п����Դ���b����B����
C��NaHS��Na2S�Ļ����Һ�д��ڵ���غ㣬����һ�����ڣ�c��Na+��+c��H+��=c��OH-��+c��HS-��+2c��S2-������C��ȷ��
D������Һ��c��OH-��=10d-14mol/L��ˮ�������c��H+��=c��OH-��=10d-14mol/L����D����
��ѡC��
���� ���⿼����Һ������Ũ�ȵĹ�ϵ����Ŀ�Ѷ��еȣ����ݵ���ƽ�ⳣ���жϸ����������ǿ��Ϊ���ؼ���ע�����յ���غ㡢�����غ㡢�ε�ˮ��ԭ�����ж�����Ũ�ȴ�С�е�Ӧ�÷�����

 ��ʦ�㲦��ϵ�д�
��ʦ�㲦��ϵ�д�| A�� | ���������� | B�� | ˮ | C�� | ֬�������� | D�� | ������ |
| A�� | HCl MgCl2 NH4Cl | B�� | H2O Na2O ? CO2 | ||
| C�� | NH3 H2O CO2 | D�� | CaCl2 ? NaOH H2O |
| �¶�/�� | 700 | 800 | 830 | 1000 | 1200 |
| ƽ�ⳣ�� | 1.7 | 1.1 | 1.0 | 0.6 | 0.4 |
| A�� | 4 sʱ������c��Y��=0.76 mol/L | |
| B�� | 830���ƽ��ʱ��X��ת����Ϊ80% | |
| C�� | ��Ӧ��ƽ��������¶ȣ�ƽ�������ƶ� | |
| D�� | 1200��ʱ��ӦR��g��+Q��g��?X��g��+Y��g����ƽ�ⳣ��K=0.4 |
| A�� | ���Ʊ�С�մ�ķ���ʽΪ��NaCl+NH4HCO3��NaHCO3��+NH4Cl | |
| B�� | ĸҺ��ͨ��İ�����HCO3-��Ӧ��NH3+HCO3-��CO32-+NH4+ | |
| C�� | ��ʳ����Ϊ������Һ��Cl-��Ũ�� | |
| D�� | �����֪�¶Ƚϵ�ʱ���Ȼ�淋��ܽ�ȱ��Ȼ��ƵĴ� |
| A�� | ���ۺ�ɱ���������� | B�� | �����ȷ�ֹĥ�� | ||
| C�� | ����Ӳ�ȷ�ֹײ�� | D�� | ��ֹ������������ |

 ��
��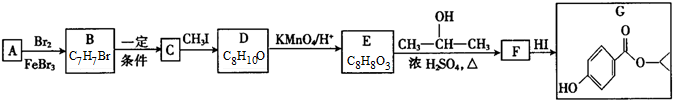
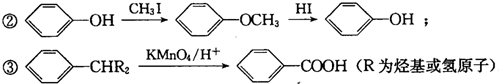
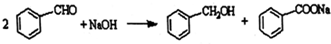
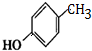 G�Ĺ���������Ϊ�ǻ�������
G�Ĺ���������Ϊ�ǻ�������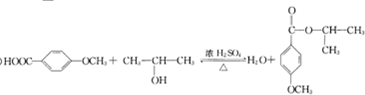
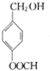
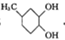

 ��
��