��Ŀ����
2����һ���������ķ�����ɽ�һ�����ʹ��������������������ʣ���CH3-CH3 ��CH2=CH2 ��
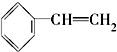 ��
��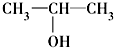 ��
��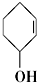 ��
��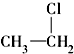 ��
��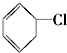 ��
��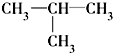 ��CH3-CH=CH2 ��10��
��CH3-CH=CH2 ��10��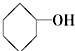 ��11��
��11��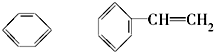 ⑫CH3Cl
⑫CH3Cl���У�
��1��������״��������Т٢ڢܢޢ��⑫������ţ���ͬ����
��2������±�������Тޢ�⑫��
��3������������������Тܢݢޢߢ�⑫��
��4�����ڷ��������Т�⑪��
������ij������ʽΪCnH2n-2��������KMnO4��Һ������õ�

�Ļ���������Ľṹ��ʽΪ
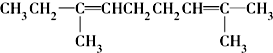 ��
��
���� ��һ����1�������в�����̼�����л���������״�����
��2��±������������ԭ�ӱ�±��ԭ��ȡ����õ����л��
��3��������������������õ����л���л��ﺬ̼�����������Ԫ�أ�
��4�����������������������ڷ�������
������ij��������KMnO4��Һ�������õ�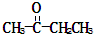 ��
��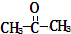 ��
�� �Ļ�����2��C=C���Դ˽��н��
�Ļ�����2��C=C���Դ˽��н��
��� �⣺��һ����1���٢ڢܢޢ��⑫����״�������������о�����̼�����ʴ�Ϊ���٢ڢܢޢ��⑫��
��2���ޢ�⑫������C��H����Ԫ��֮�⣬������ԭ�ӣ�����±�������ʴ�Ϊ���ޢ�⑫��
��3���ܢݢޢߢ�⑫����̼�������O��ClԪ�أ���������������ʴ�Ϊ���ܢݢޢߢ�⑫��
��4����⑪���������ڷ��������ʴ�Ϊ����⑪��
������ij������ʽΪCnH2n-2��������KMnO4��Һ�������õ�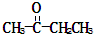 ��
��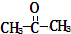 ��
�� �Ļ�������л�����Ӻ�2��C=C��������ʽΪC11H20�Ľṹ��ʽΪ
�Ļ�������л�����Ӻ�2��C=C��������ʽΪC11H20�Ľṹ��ʽΪ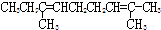 ���ʴ�Ϊ��
���ʴ�Ϊ��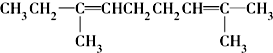 ��
��
���� ���⿼�����л���ķ�����ϩ�������ʣ����չ����������ʵĹ�ϵΪ���Ĺؼ�������ϩ�����ʵĿ��飬ע��C=C�ϲ���H����Ϊͪ����H����Ϊ-COOH�ϻ�������������������������ȷ���з��࣮

��ϰ��ϵ�д�
 ��ְٷְټ���ϵ�д�
��ְٷְټ���ϵ�д� �����ƻ���ĩ��̶�100��ϵ�д�
�����ƻ���ĩ��̶�100��ϵ�д�
�����Ŀ
12��a g CuO��Al2O3��MgO��ɵĻ����ȫ������20mLij����Һ�У���Ӧ����������Һ�м���10mL����������Һ���õ��ij�������������ж�Ӧ��ϵ��ȷ���ǣ�������
| ѡ�� | ����Һ | c��NaOH�� |
| A | pH=1������ | 0.4mol/L |
| B | pH=1������ | 0.4mol/L |
| C | 0.1mol/L������ | 0.2mol/L |
| D | 0.1mol/L������ | 0.2mol/L |
| A�� | A | B�� | B | C�� | C | D�� | D |
13������˵����ȷ���ǣ�������
| A�� | ԭ�Ӻ˶��������Ӻ����ӹ��� | |
| B�� | ��������ͬ�������仯ѧ���ʲ�һ����ͬ | |
| C�� | ij���������������8�����ӣ���һ����ϡ������Ԫ�ص�ԭ�� | |
| D�� | ԭ�ӵ��ӹ���Ϊns2np6��Ϊϡ������Ԫ�� |
10��������һ�ֹ�ҵԭ�ϣ���ҵ�����÷�Ӧ3Cl2+2NH3�TN2+6HCl��������ܵ��Ƿ�©��������˵��������ǣ�������
| A�� | ���ܵ�©��������������� | B�� | �÷�Ӧ������������ǿ������ | ||
| C�� | �÷�Ӧ���ڸ��ֽⷴӦ | D�� | ����1 mol N2��6 mol����ת�� |
17�����¼����������£�ij��Ӧ��ƽ�ⳣ��K=$\frac{[C{O}_{2}][{H}_{2}]}{[CO][{H}_{2}O]}$������ʱ���¶����ߣ�H2Ũ�ȼ�С������˵����ȷ���ǣ�������
| A�� | �����£������������Ϊԭ��2����CO��ƽ��Ũ�ȱ�Ϊԭ����$\frac{1}{2}$ | |
| B�� | ���º����£�����ѹǿ��H2Ũ��һ����С | |
| C�� | �����¶ȣ�����Ӧ���ʼ�С���淴Ӧ�������� | |
| D�� | �÷�Ӧ��ѧ����ʽΪCO2+H2?CO+H2O��H��0 |
7�������£�pH=a��pH=b������NaOH��Һ����֪b=a+2����������Һ�������Ϻ�������Һ��pH�ӽ��ڣ�������
| A�� | a-lg2 | B�� | b-lg2 | C�� | a+lg2 | D�� | b+lg2 |
11����ҵ�ϻ�������ij�Ͻ���ϣ���Ҫ��Fe��Cu��Co��Li�ȣ���֪Co��Fe�����еȻ��ý������Ĺ����������£�
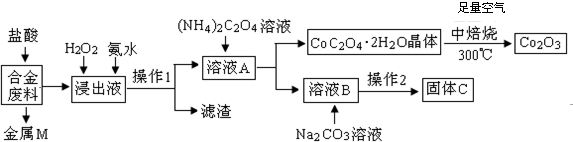
��1������MΪCu������1Ϊ���ˣ�
��2������H2O2�������ǣ������ӷ���ʽ��ʾ��2Fe2++H2O2+2H+=2Fe3++2H2O�����백ˮ�������ǵ���PHʹFe3+ȫ��ת��Ϊ��������������
��3����CoC2O4•2H2Oת��ΪCo2 O3�Ļ�ѧ����ʽ��4��CoC2O4?2H2O��+3O2$\frac{\underline{\;����\;}}{\;}$2Co2O3+8H2O+8CO2��
��4��֪Li2CO3����ˮ����ˮ��Һ�ʼ��ԣ��䱥����Һ��Ũ�����¶ȹ�ϵ���±�������2�У�����Ũ���������ȹ��ˣ���ԭ���Ǽ���Li2CO3���ܽ���ʧ���ڳ��������£�����Li2CO3��Һ������Ũ���ɴ�С������˳��Ϊc��Li+����c��CO32-����c��OH-����c��HCO3-����c��H+����
��5���ö��Ե缫�������Li2CO3��ȡﮣ����������������壬�������ĵ缫��ӦʽΪ2CO32--4e-=O2��+CO2����

��1������MΪCu������1Ϊ���ˣ�
��2������H2O2�������ǣ������ӷ���ʽ��ʾ��2Fe2++H2O2+2H+=2Fe3++2H2O�����백ˮ�������ǵ���PHʹFe3+ȫ��ת��Ϊ��������������
��3����CoC2O4•2H2Oת��ΪCo2 O3�Ļ�ѧ����ʽ��4��CoC2O4?2H2O��+3O2$\frac{\underline{\;����\;}}{\;}$2Co2O3+8H2O+8CO2��
��4��֪Li2CO3����ˮ����ˮ��Һ�ʼ��ԣ��䱥����Һ��Ũ�����¶ȹ�ϵ���±�������2�У�����Ũ���������ȹ��ˣ���ԭ���Ǽ���Li2CO3���ܽ���ʧ���ڳ��������£�����Li2CO3��Һ������Ũ���ɴ�С������˳��Ϊc��Li+����c��CO32-����c��OH-����c��HCO3-����c��H+����
| �¶�/�� | 10 | 30 | 60 | 90 |
| Ũ��/mol?L-1 | 0.21 | 0.17 | 0.14 | 0.10 |
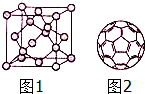 ̼��������������Ĺ�ϵ�dz����У���̼���ʼ��仯������о�������Ҫ���壮
̼��������������Ĺ�ϵ�dz����У���̼���ʼ��仯������о�������Ҫ���壮 2Na2CO3+C��CO32-�Ŀռ乹��Ϊƽ���������Σ���������������
2Na2CO3+C��CO32-�Ŀռ乹��Ϊƽ���������Σ���������������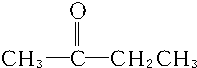 C��CH2�TCH-CH2CH3��CH3CH�TCH-CH3��
C��CH2�TCH-CH2CH3��CH3CH�TCH-CH3��