��Ŀ����
13������ع㷺Ӧ���ڻ�϶�������ϵͳ���缫������Ni��OH��2��̼�ۡ���������Ϳ�����������Ƴɣ����ڵ��ʹ�ú�缫���϶Ի�����Σ����ij��ȤС��Ըõ�ص缫���Ͻ�����Դ�����о������ʵ��·�����£�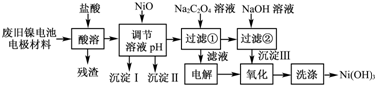
��֪����NiCl2������ˮ��Fe3+��������Ni2+��
����֪ʵ���¶�ʱ���ܽ�ȣ�NiC2O4��NiC2O4•H2O��NiC2O4•2H2O
�ش��������⣺
��1����NiO������Һ��pH�����������ijɷ�ΪFe��OH��3��Al��OH��3���ѧʽ����
��2��д������Na2C2O4��Һ�ķ�Ӧ�Ļ�ѧ����ʽ��NiCl2+Na2C2O4+2H2O�TNiC2O4•2H2O��+2NaCl��
��3�������Һʱ�Ļ�ѧ����ʽΪ2NaCl+2H2O$\frac{\underline{\;ͨ��\;}}{\;}$2NaOH+Cl2��+H2������������Һʱ��������������ķ�����ʪ��ĵ��۵⻯����ֽ��
��4��д��������Ӧ�����ӷ���ʽ��2Ni��OH��2+2OH-+Cl2�T2Ni��OH��3+2Cl-��
��5����μ���Ni��OH��3��ϴ�Ӹɾ���ȡ���һ��ϴ��Һ�������Թ��У���������AgNO3��Һ�����ް�ɫ�������ɣ�֤��������ϴ�Ӹɾ���
���� ���̷�����֪���Ͼ�����ص缫������Ni��OH��2��̼�ۡ���������Ϳ�����������Ƴɣ����ܹ��˵õ���Һ������NiO������ҺPH���������ӣ����˵õ���Һ�м����������Һ���Ȼ�����Ӧ���ɲ��������壬���˵õ���Һ��������������Һ�õ����������ɵ�������������Һ������������ͼ�ʹ��������������Ϊ2Ni��OH��3���ɴ˷������
��� �⣺��1����NiO������Һ��pH��������Fe��OH��3������Ȼ������Al��OH��3�������ʴ�Ϊ��Fe��OH��3��Al��OH��3��
��2��NiCl2����Na2C2O4��Ӧ����NiC2O4•2H2O��NaCl����Ӧ�Ļ�ѧ����ʽΪNiCl2+Na2C2O4+2H2O�TNiC2O4•2H2O��+2NaCl���ʴ�Ϊ��NiCl2+Na2C2O4+2H2O�TNiC2O4•2H2O��+2NaCl��
��3����ҺΪ�Ȼ�����Һ������Ȼ�����Һ�Ļ�ѧ����ʽΪ��2NaCl+2H2O$\frac{\underline{\;ͨ��\;}}{\;}$2NaOH+Cl2��+H2�������ʱ��������������������ǿ�����ԣ��ʿ���ʪ��ĵ��۵⻯����ֽ���ʴ�Ϊ��2NaCl+2H2O$\frac{\underline{\;ͨ��\;}}{\;}$2NaOH+Cl2��+H2������ʪ��ĵ��۵⻯����ֽ��
��4������2�õ�Ni��OH��2�����Һ1�������������߷���������ԭ��Ӧ�����ӷ���ʽΪ��2Ni��OH��2+2OH-+Cl2�T2Ni��OH��3+2Cl-��
�ʴ�Ϊ��2Ni��OH��2+2OH-+Cl2�T2Ni��OH��3+2Cl-��
��5����©���м�����ˮ����û������ʹˮ��Ȼ���꣬�ظ�����2��3�Σ�ȡ���һ��ϴ��Һ�������Թ��У���������AgNO3��Һ�����ް�ɫ�������ɣ�֤��������ϴ�Ӹɾ����ʴ�Ϊ��ȡ���һ��ϴ��Һ�������Թ��У���������AgNO3��Һ�����ް�ɫ�������ɣ�֤��������ϴ�Ӹɾ���
���� ���⿼�黯ѧ��Ӧԭ���еĵ绯ѧ����ѧ����ʽ����д�����ӵļ���ȣ��Ѷ����У�

| A�� | KW�dz���ʱ��10-2�� | B�� | �¶Ȳ����ϡ10����pH=7 | ||
| C�� | pH=6�������� | D�� | c��H+��=10-6mol/L����ҺΪ���� |
| A�� | ˮ | B�� | ��ˮ | ||
| C�� | ��̪��Һ | D�� | ���Ը��������Һ |
| A�� | ���������ſ������ռ����� | |
| B�� | ������������ˮ����˼���Ũ��ˮ����������� | |
| C�� | �������ֱ�պ��Ũ��ˮ��Ũ����IJ���������ʱ���а��̲��� | |
| D�� | ��ʵ�����У����ü����������ƺ��Ȼ�淋Ĺ�������ķ�����ȡ���� |
| A�� | ���ԣ�H2SO4��HClO4 | B�� | ���ԣ�NaOH��KOH | ||
| C�� | �ǽ����ԣ�P��S | D�� | ��̬�⻯���ȶ��ԣ�HCl��H2S |
| A�� | ������FeSO4��Һʱ�������м���һ�������ۺ�ϡ���� | |
| B�� | �ù㷺pH��ֽ������ij��Һ��pH=13 | |
| C�� | �ü�ʽ�ζ�����ȡ20.00mL0.1000mol/LKmnO4��Һ | |
| D�� | ʵ���ҿɽ�ͭ������������ͭ���������ᷴӦ����ȡ����ͭ�Խ�ԼҩƷ��������Ⱦ |
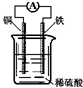
| A�� | ��װ���ܽ�����ת��Ϊ��ѧ�� | B�� | ͭ�����ܽ� | ||
| C�� | ����������ͨ����������ͭ�� | D�� | ������ӦΪFe-2e-�TFe2+ |
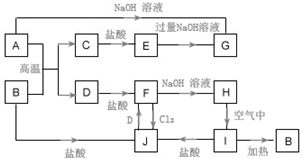 A��JΪ��ѧ��ѧ�ij������ʣ�����֮������ͼ��ʾ��ת����ϵ�����ֲ�������ȥ������֪A��DΪ�������ʣ�BΪ����ɫ��ĩ��IΪ���ɫ���壮
A��JΪ��ѧ��ѧ�ij������ʣ�����֮������ͼ��ʾ��ת����ϵ�����ֲ�������ȥ������֪A��DΪ�������ʣ�BΪ����ɫ��ĩ��IΪ���ɫ���壮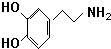 ������������е�һ���л����ʽ�����Ͱ���
������������е�һ���л����ʽ�����Ͱ���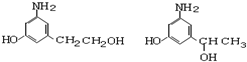 ��
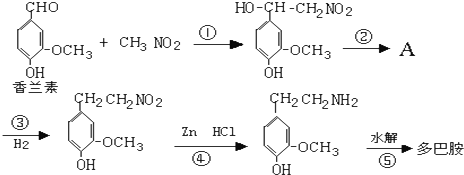
�� 
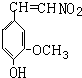 ��
��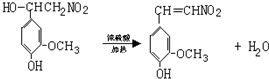 ���ݣ�
���ݣ�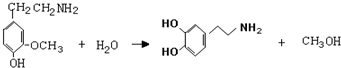 ��
��