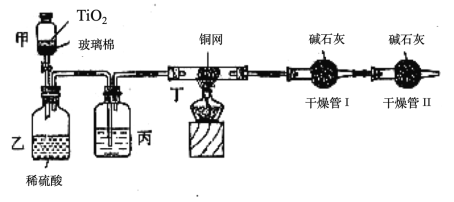
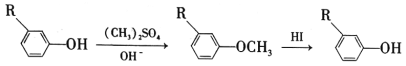
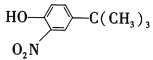
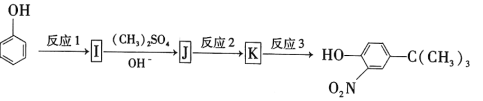
��Ŀ����
����Ŀ��ij�л����A��Ϊͬ���칹�壬���ⶨ���ǵ���Է�������С��100����1mol����O2�г��ȼ�յõ������ʵ�����CO2��H2O (g) ��ͬʱ����112L O2����״�����������������½�1mol����ȫˮ���������1mol�Һ�1mol����������һ�������£������Ա�����������Ϊ�ҡ�

��������ײⶨ���ڼ�A�Ľṹ�ж�����C=O˫����C-O������B��HIO4���ڲ�����ʱֻ����һ�ֲ���C������Ϊ����ط�Ӧ����Ϣ��ת����ϵ��
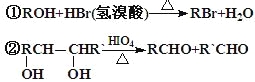
�� ��ȷ����д���ķ���ʽ_______�������ͬ�����ʵ�ͬ���칹�干��____�֣������ף���
�� E��F �ķ�Ӧ����Ϊ_________��Ӧ��
�� A�Ľṹ��ʽΪ_________��G �Ľṹ��ʽΪ_________��
�� B��D�ķ�Ӧ��ѧ����ʽΪ_______________________________��
�� д��C���������½��з�Ӧ�Ļ�ѧ����ʽ___________________��
���𰸡�C4H8O2 4 ��ȥ��Ӧ ![]()
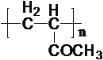

![]()
��������
�����������£�1mol����ȫˮ��Ϊ1mol�Һ�1mol�����ұ����Ա�����Ϊ�ң����Ϊ������Ϊ���ᣬ��Ϊ�������ҡ�����̼ԭ�Ӹ�����ͬ���Ȼ����ǻ��ĸ���Ҳ��ͬ������1mol����O2�г��ȼ�յõ������ʵ�����CO2��H2O�����ڼ����У�C��H�ĸ�����Ϊ1��2�����ΪһԪ����������ΪһԪ�������ᣬ��ΪһԪ���ʹ�������1mol����ȫȼ�գ�����5molO2��![]() =5mol������ķ���ʽΪCnH2nO2����ȼ�շ�ӦΪ��
=5mol������ķ���ʽΪCnH2nO2����ȼ�շ�ӦΪ��![]() ������
������![]() �����n=4����ķ���ʽΪC4H8O2��������Ϊ98��С��100�������Ϊ����������CH3COOCH2CH3����Ϊ����CH3COOH����Ϊ�Ҵ�CH3CH2OH��CΪCH3CHO��B��HIO4��Ӧ����C����BΪ
�����n=4����ķ���ʽΪC4H8O2��������Ϊ98��С��100�������Ϊ����������CH3COOCH2CH3����Ϊ����CH3COOH����Ϊ�Ҵ�CH3CH2OH��CΪCH3CHO��B��HIO4��Ӧ����C����BΪ ��A��H2��Ӧ����B����A�ͼ�Ϊͬ���칹�壬A�к���C=O˫����C-O��������AΪ
��A��H2��Ӧ����B����A�ͼ�Ϊͬ���칹�壬A�к���C=O˫����C-O��������AΪ �����ݷ�Ӧ�٣������Ƴ�EΪ
�����ݷ�Ӧ�٣������Ƴ�EΪ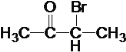 ��FΪ
��FΪ ��GΪ
��GΪ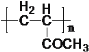 ��DΪ
��DΪ ��(1)����������Ϊ���������������ʽΪC4H8O2����ͬ�����ʵ�ͬ���칹���м�����������������������������������������£�����4��ͬ���칹�壻
��(1)����������Ϊ���������������ʽΪC4H8O2����ͬ�����ʵ�ͬ���칹���м�����������������������������������������£�����4��ͬ���칹�壻
(2) EΪ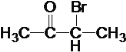 ��FΪ
��FΪ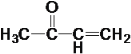 ����E��F�ķ�Ӧ����Ϊ��ȥ��Ӧ��
����E��F�ķ�Ӧ����Ϊ��ȥ��Ӧ��
(3) A�Ľṹ��ʽΪ ��G�Ľṹ��ʽΪ
��G�Ľṹ��ʽΪ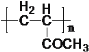 ��
��
(4) BΪ ��DΪ
��DΪ ����B��D�Ļ�ѧ����ʽΪ
����B��D�Ļ�ѧ����ʽΪ ��
��
(5)CΪCH3CHO����������Ӧ�ķ���ʽΪ![]() ��
��

 ��������ϵ�д�
��������ϵ�д� ���ɶ���ܲ��¿�ֱͨ�߿�ϵ�д�
���ɶ���ܲ��¿�ֱͨ�߿�ϵ�д�