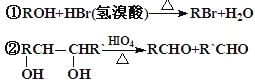
��Ŀ����
����Ŀ�������仯�����������ҵ������������ҪӦ�á���ش��������⣺
(1)��ͼ��N2(g)��H2(g)��NH3(g)֮��ת����������ϵͼ����
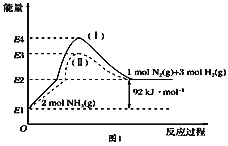
��N2(g)��H2(g)��Ӧ����NH3(g)���Ȼ�ѧ����ʽΪ___________________.
�ڹ���(��)����(��)�ķ�Ӧ��________(���ͬ����ͬ��).
��ij�¶��£���1 L���º��������г���1molN2��3 mol H2����������Ӧ��10 min�ﵽƽ�⣬��ʱ������ѹǿ��Ϊԭ����7/8.
a.�ù��̵�ƽ�ⳣ���ı���ʽΪ____________.
b.N2��ƽ��ת����Ϊ________.
c.��ʱ�����������¶Ⱥ�������䣬�������ټ���2.25 molN2��0.5 mol NH3����ƽ��________(�����������)�ƶ�.
(2)��NH3�������������������Ⱦ����֪��
��Ӧ��4NH3(g)��3O2(g)![]() 2N2(g)��6H2O(g) ��H1��a kJ��mol��1 ƽ�ⳣ��ΪK1
2N2(g)��6H2O(g) ��H1��a kJ��mol��1 ƽ�ⳣ��ΪK1
��Ӧ��N2(g)��O2(g)![]() 2NO(g) ��H2��b kJ��mol��1 ƽ�ⳣ��ΪK2
2NO(g) ��H2��b kJ��mol��1 ƽ�ⳣ��ΪK2
��Ӧ��4NH3(g)��6NO(g)![]() 5N2(g)��6H2O(g) ��H3��c kJ��mol��1 ƽ�ⳣ��ΪK3
5N2(g)��6H2O(g) ��H3��c kJ��mol��1 ƽ�ⳣ��ΪK3
��Ӧ���е�b��_____(�ú�a��c�Ĵ���ʽ��ʾ)��K3=_____(��K1��K2��ʾ).��Ӧ���еĦ�S______(�>����<������)0.
(3)�ں��ݵ��ܱ����У�����һ������NH3��NO����������Ӧ��ò�ͬ�¶��·�Ӧ��ϵ��NH3��ת����(��)��ѹǿp�Ĺ�ϵ��ͼ��ʾ��
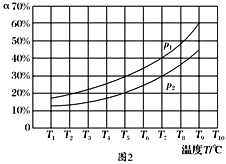
�ٷ�����p1________p2.(�>����<������)
�����������У�������Ϊ�жϷ�Ӧ���Ѿ��ﵽƽ��״̬�ı�־����________(�����).
a��N2��Ũ�Ȳ��ٸı� b������6 mol N��H����ͬʱ����6 mol H��O���γ�
c��������ѹǿ���ٱ仯 d�����������ܶȱ��ֲ���
���𰸡�N2(g)��3H2(g)![]() 2NH3(g) ��H����92 kJ��mol��1 ��ͬ K=c2(NH3)/��c(N2)c3(H2)�� 25% �� (a-c)/3
2NH3(g) ��H����92 kJ��mol��1 ��ͬ K=c2(NH3)/��c(N2)c3(H2)�� 25% �� (a-c)/3 ![]() > < bd
> < bd
��������
��1���پ�ͼ��֪2molNH3�ֽ�õ�1molN2��3molH2������92kJ/mol�����������N2(g)��H2(g)��Ӧ����NH3(g)���Ȼ�ѧ����ʽΪN2(g)��3H2(g) ![]() 2NH3(g) ��H����92 kJ��mol��1��
2NH3(g) ��H����92 kJ��mol��1��
�ڸ��ݸ�˹���ɣ���Ӧ��ֻ����ʼ״̬������״̬�йأ�������أ�������������ʼ״̬������״̬��ͬ�������Ӧ����ͬ��
�ۿ��Ը�������ʽȥ��⣬��ת��xmol/LN2��
N2(g)��3H2(g) ![]() 2NH3(g)
2NH3(g)
�� 1 3 0
ת x 3x 2x
ƽ 1-x 3-3x 2x
���ݴ�ʱ������ѹǿ��Ϊԭ����7/8������ʽ��![]() ����x=0.25mol/L��
����x=0.25mol/L��
a. K= ![]() ��
��
b. N2��ƽ��ת����Ϊ0.25/1��100��=25����
c. ƽ��ʱc(N2)=0.75mol/L��c(H2)=2.25mol/L��c(NH3)=0.5mol/L��K=![]() =0.029���������ټ���2.25 molN2��0.5 mol NH3����c(N2)=3mol/L��c(H2)=2.25mol/L��c(NH3)=1mol/L��Q=
=0.029���������ټ���2.25 molN2��0.5 mol NH3����c(N2)=3mol/L��c(H2)=2.25mol/L��c(NH3)=1mol/L��Q= ![]() =0.029�����Q=K��ƽ�ⲻ�ƶ���
=0.029�����Q=K��ƽ�ⲻ�ƶ���
��2�����ݸ�˹���ɣ��跴Ӧ�����������ֱ�ΪA��B��C����B=(A-C)/3�����b=(a-c)/3������c=a-3b�����Կ�֪K3=K1/K23������4NH3(g)��6NO(g)![]() 5N2(g)��6H2O(g)��֪�÷�Ӧ�����ʾ�Ϊ���壬�������������������Ҷȱ�ʦ�S>0��
5N2(g)��6H2O(g)��֪�÷�Ӧ�����ʾ�Ϊ���壬�������������������Ҷȱ�ʦ�S>0��
��3������4NH3(g)��6NO(g)![]() 5N2(g)��6H2O(g)��֪�÷�Ӧ������������ڷ�Ӧǰ��������������¶Ȳ��䣬NH3��ת����(��)Խ��˵��ѹǿԽС�����p1<p2��a��N2��Ũ�Ȳ��ٸı䣬˵�������Ũ�ȶ����䣬���������Ϊ�ж�ƽ��ı�־����ȷ��b������6 mol N��H����ͬʱ����6 mol H��O���γɣ����ݷ�Ӧ�ص㣬���߶���ʾ����Ӧ���ʣ��������c�����ڷ�Ӧǰ������������仯����������ѹǿ���ٱ仯�����ж�ƽ�⣬��ȷ��d�����������ܶȦ�=m/V�����ڸ���ֶ������壬��Ӧǰ�����������������䣬�������㶨��������䣬����ܶ�ʼ���Ǹ���ֵ�����ʴ�ѡbd��
5N2(g)��6H2O(g)��֪�÷�Ӧ������������ڷ�Ӧǰ��������������¶Ȳ��䣬NH3��ת����(��)Խ��˵��ѹǿԽС�����p1<p2��a��N2��Ũ�Ȳ��ٸı䣬˵�������Ũ�ȶ����䣬���������Ϊ�ж�ƽ��ı�־����ȷ��b������6 mol N��H����ͬʱ����6 mol H��O���γɣ����ݷ�Ӧ�ص㣬���߶���ʾ����Ӧ���ʣ��������c�����ڷ�Ӧǰ������������仯����������ѹǿ���ٱ仯�����ж�ƽ�⣬��ȷ��d�����������ܶȦ�=m/V�����ڸ���ֶ������壬��Ӧǰ�����������������䣬�������㶨��������䣬����ܶ�ʼ���Ǹ���ֵ�����ʴ�ѡbd��

 ��У����ϵ�д�
��У����ϵ�д�����Ŀ���о�NO2��SO2 ��CO�ȴ�����Ⱦ����Ĵ���������Ҫ���塣
��1��һ�������£���2molNO��2molO2���ں����ܱ������з������·�Ӧ��2NO(g)+O2(g)![]() 2NO2(g)�����и�����˵����Ӧ�ﵽƽ��״̬����_____________��
2NO2(g)�����и�����˵����Ӧ�ﵽƽ��״̬����_____________��
A.��ϵѹǿ���ֲ���
B.���������ɫ���ֲ���
C.NO��O2�����ʵ���֮�ȱ��ֲ���
D.ÿ����1 molO2ͬʱ����2 molNO
��2��CO�����ںϳɼ״���һ���¶��£������Ϊ2L���ܱ������м���CO��H2��������ӦCO(g)+2H2(g)![]() CH3OH(g)����ƽ����ø����Ũ�ȣ�
CH3OH(g)����ƽ����ø����Ũ�ȣ�
���� | CO | H2 | CH3OH |
Ũ�ȣ�mol/L�� | 0.9 | 1.0 | 0.6 |
�ش��������⣺
�ٻ�������ƽ����Է�������=_________________��
��ƽ�ⳣ��K=__________________��
�������������ѹ��Ϊ1L���������㣬Ԥ����ƽ����c(H2)��ȡֵ��Χ��__________��
��������������䣬�ٳ���0.6molCO��0.4molCH3OH����ʱv��______v�������������������=������
����Ŀ����Դ������������������Դ���õ��ǵ����������Ż��⡣�������ѧ��ѧ֪ʶ�ش��������⣺
��1�������ϰ�װ��ת��������ʹ����β���е���Ҫ��Ⱦ�CO��NOx��̼�⻯����������Ӧ�����������ʣ���������β����Ⱦ��
��֪��N2(g) + O2(g)��2NO(g) ��H��+180.5 kJ �� mol��1��
2C(s)+ O2(g)��2CO(g) ��H����221.0 kJ �� mol��1��
C(s)+ O2(g)��CO2(g) ��H����393.5 kJ �� mol��1��
��β��ת����Ӧ2NO(g) +2CO(g)��N2(g)+2CO2(g)����H��________________��
��2������β�������Ƕ�CO�ĺ�����������ȼ�ϵ��Ϊ����ԭ������װ������ͼ��ʾ���õ���е����Ϊ�����ƣ������ƣ�����O2-�����ڹ�������������ƶ���

����˵������ȷ����_____________(����ĸ���)��
A�������ĵ缫��ӦʽΪ��CO + O2���D2e����CO2
B������ʱ�����ɵ缫aͨ������������缫b
C������ʱ�缫b��������O2���ɵ缫aͨ�����������缫bǨ��
D����������ͨ���ĵ���Խ��β����CO�ĺ���Խ��
��3��ij���᳧���ü״�������ˮ����һ�������£����ˮ�м���CH3OH����HNO3��ԭ��N2�����÷�Ӧ����32 g CH3OHת��6 mol���ӣ���μӷ�Ӧ�Ļ�ԭ���������������ʵ���֮��Ϊ______________��
��4��ú�ļ��Һ������ת��ΪCO��H2�����ڴ��������ºϳɼ״�������һ���¶��£���1 L�ܱ������м���CO��H2��������ӦCO(g)+2H2(g)![]() CH3OH(g)����10 min��Ӧ�ﵽƽ��ʱ��ø���ֵ�Ũ�����£�
CH3OH(g)����10 min��Ӧ�ﵽƽ��ʱ��ø���ֵ�Ũ�����£�
�� �� | CO | H2 | CH3OH |
Ũ��/(mol��L��1) | 1.2 | 1.0 | 0.6 |
�ٸ÷�Ӧ��ƽ�ⳣ������ʽΪ��K��_____________________��
�ڸ�ʱ���ڷ�Ӧ������(H2)��_________________��
��ƽ��ʱCO��ת����Ϊ_________________(����1λС��)��