��Ŀ����
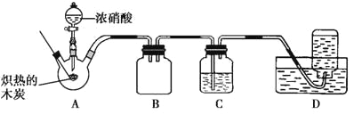
����Ŀ����ͼ��ʵ�����Ʊ�����������һϵ�����ʵ���װ��(�г��豸�� A �оƾ�������)��
(1)ʵ���������������ӷ���ʽΪ_____��
(2)װ�� B �б���ʳ��ˮ��������_____����д��װ�� B ����һ������_______________________________��
(3)װ�� C ��ʵ��Ŀ������֤�����Ƿ����Ư���ԣ� Ϊ�� C ���������������η������ʵ������_______________(����)��
��� | a | b | c | d |
�� | �������ɫ���� | �������ɫ���� | ʪ�����ɫ���� | ʪ�����ɫ���� |
�� | ��ʯ�� | �轺 | Ũ���� | ��ˮ�Ȼ��� |
�� | ʪ�����ɫ���� | ʪ�����ɫ���� | �������ɫ���� | �������ɫ���� |
(4)���װ�� D��E ��Ŀ���DZȽ��ȡ��塢��ķǽ���������Ӧһ��ʱ���������װ�� D ��������Һ����װ�� E ���������������۲쵽��������_____����������˵����ķǽ�����ǿ�ڵ⣬ԭ����_________��
(5)װ�� F �������������ӷ�Ӧ����ʽ��_____��
���𰸡�MnO2 + 2Cl-+ 4H+=Mn2++ Cl2��+ 2H2O ��ȥ�����е��Ȼ��� ���ʵ�����ʱ C ���Ƿ������� d ��Һ�ֲ㣬�ϲ�Ϊ�Ϻ�ɫ���²�ӽ���ɫ ����������ʱ��Ҳ�����û����ⵥ�� Cl2 + SO32- + H2O====2Cl- + SO42- + 2H+
��������
��1��ʵ���������������ӷ���ʽΪMnO2+4H++2Cl-![]() Mn2++Cl2��+2H2O��
Mn2++Cl2��+2H2O��
��2��װ��B�б���ʳ��ˮ�������dz�ȥ�����е��Ȼ��⣬���ʵ�������װ��C������������װ��B�г���©���е�Һ��ͻ�������
��3��װ��C��ʵ��Ŀ������֤�����Ƿ����Ư���ԣ���֤�����Ƿ����Ư���ԣ�Ҫ��֤����������Ư���ԣ�ʪ�����ɫ�����У�������ˮ��Ӧ���ɴ��������Ư������
��4���ȵ��ʺ��廯�Ʒ�Ӧ�����嵥�ʣ��嵥�ʺ͵⻯�ط�Ӧ���ɵⵥ�ʣ��ⵥ�����ڱ����Ϻ�ɫ�����۲쵽�������ǣ�E����Һ��Ϊ���㣬�ϲ㣨���㣩Ϊ�Ϻ�ɫ���²�ӽ���ɫ����������ǿ�����ԣ������������ܹ��������������ɵⵥ�ʣ����Ը�������˵����ķǽ�����ǿ�ڵ���
��5��װ��F�����������ն������������ֹ��Ⱦ��������Ӧ�����ӷ���ʽΪ��Cl2+SO32-+H2O=2Cl- +SO42-+2H+��
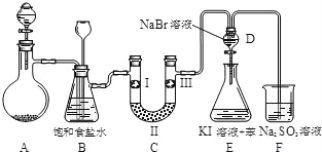
��1��װ��A�еĹ���ҩƷ��MnO2����Һ©����ʢ�ŵ���Ũ���ᣬMnO2��Ũ���ᷢ����Ӧ�Ļ�ѧ����ʽΪ��4HCl+MnO2![]() MnCl2+Cl2��+2H2O�����ӷ���ʽ��MnO2+4H++2Cl-
MnCl2+Cl2��+2H2O�����ӷ���ʽ��MnO2+4H++2Cl-![]() Mn2++Cl2��+2H2O���ʴ�Ϊ��MnO2+4H++2Cl-
Mn2++Cl2��+2H2O���ʴ�Ϊ��MnO2+4H++2Cl-![]() Mn2++Cl2��+2H2O��
Mn2++Cl2��+2H2O��
��2��װ��B�б���ʳ��ˮ�������dz�ȥ�����е��Ȼ��⣬���ʵ�������װ��C������������װ��B�г���©���е�Һ��ͻ�����������װ��B�����ü��ܳ�ȥ�����е��Ȼ��⣬���ܼ��ʵ�����ʱC���Ƿ����������ʴ�Ϊ����ȥ�����е��Ȼ��⣬���ʵ�����ʱC���Ƿ���������
��3��װ��C��ʵ��Ŀ������֤�����Ƿ����Ư���ԣ���֤�����Ƿ����Ư���ԣ�Ҫ��֤����������Ư���ԣ�ʪ�����ɫ�����У�������ˮ��Ӧ���ɴ��������Ư���ԣ�I����ʪ�����ɫ��������II��Ӧ�����̬������Ҳ�������������III�����ø������ɫ����������C��I��II��III���η���ʪ�����ɫ��������ˮ�Ȼ��ơ��������ɫ��������ѡd���ʴ�Ϊ��d��
��4����������װ��D��������Һ����װ��E�У��ȵ��ʺ��廯�Ʒ�Ӧ�����嵥�ʣ��嵥�ʺ͵⻯�ط�Ӧ���ɵⵥ�ʣ��ⵥ�����ڱ����Ϻ�ɫ�����۲쵽�������ǣ�E����Һ��Ϊ���㣬�ϲ㣨���㣩Ϊ�Ϻ�ɫ���²�ӽ���ɫ����������ǿ�����ԣ������������ܹ��������������ɵⵥ�ʣ����Ը�������˵����ķǽ�����ǿ�ڵ⣻
�ʴ�Ϊ����Һ�ֲ㣬�ϲ�Ϊ�Ϻ�ɫ���²�ӽ���ɫ������������ʱ��Ҳ�����û����ⵥ����
��5��װ��F�����������ն������������ֹ��Ⱦ��������Ӧ�����ӷ���ʽΪ��Cl2+SO32-+H2O=2Cl- +SO42-+2H+���ʴ�Ϊ��Cl2+SO32-+H2O=2Cl- +SO42-+2H+��