��Ŀ����
����Ŀ��ij��ѧѧϰС���������װ�ã���Ũ������ľ̿�ķ�Ӧ����̽����
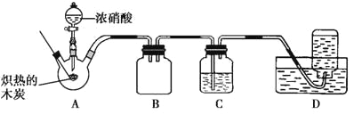
��ش��������⣺
(1)����װ�������Ժ�ȼ�ճ��е�ľ̿�ھƾ����ϼ���������״̬������������ƿ�У�������ƿ���� �μ�Ũ���ᡣ���Թ۲쵽������ƿ���������ɫΪ______������������Ļ�ѧ����ʽ��______��
(2)װ�� C ��ʢ��������Ũ Ba(OH)2 ��Һ����Ӧһ��ʱ���ɹ۲쵽 C �г��ְ�ɫ�������ð�ɫ����Ϊ__(д��ѧʽ)��
(3)װ�� B ��������______��
(4)װ�� D ���ռ�������ɫ���壬����ͬѧ��Ϊ�� NO������ͬѧ��Ϊ�� O2�����жԸ�����ļ��鷽�����ʵ���_____(����ĸ����)��
A.���ڹ۲�װ�� D �м���ƿ���������ɫ�仯
B.��ʪ�����ɫʯ����ֽ���뼯��ƿ�ڣ��۲�ʯ����ֽ�Ƿ���
C.�����ǵ�ľ�����뼯��ƿ�ڣ��۲�ľ���Ƿ�ȼ
���𰸡�����ɫ 4HNO3(Ũ)+C=CO2��+4NO2��+2H2O BaCO3 ������ A C
��������
��1�����ȵ�ľ̿��Ũ���ᷴӦ���ɶ�����̼�ͺ���ɫ�Ķ���������
��2��װ��C��ʢ��������ŨBa(OH)2��Һ��������̼ͨ�����У�������ɫ��̼�ᱵ������
��3������װ��ͼ����֪����װ��B������Ϊ������ȫƿ����ֹ��Һ������
��4������NO��O2�����ʽ����жϡ�
��1�����ȵ�ľ̿��Ũ���ᷴӦ���ɶ�����̼�ͺ���ɫ�Ķ������������Կɹ۲쵽������ƿ���������ɫΪ����ɫ����Ӧ����ʽΪ��C+4HNO3(Ũ)=4NO2��+CO2��+2H2O���ʴ�Ϊ������ɫ��C+4HNO3(Ũ)=4NO2��+CO2��+2H2O��
��2��װ��C��ʢ��������ŨBa(OH)2��Һ��������̼ͨ�����У�������ɫ��̼�ᱵ�������ʴ�Ϊ��BaCO3��
��3������װ��ͼ����֪����װ��B������Ϊ������ȫƿ����ֹ��Һ�������ʴ�Ϊ����������
��4��A.�������ƿ�ڵ�������NO��NO���ױ�����ΪNO2��ͨ�����ڹ۲���Կ�������ɫ����A���ʣ�
B.NO��O2�����������ԣ�����ʹʪ�����ɫʯ����ֽ��죬��B�����ʣ�
C.�������ǵ�ľ�����뼯��ƿ�ڣ��۲�ľ���Ƿ�ȼ�������жϼ���ƿ���Ƿ�����������C���ʡ�
�ʴ�Ϊ��AC��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ�����г�ȥ���ʵķ�����ȷ����
���� | ���� | �Լ� | ��Ҫ���� | |
A | NaHCO3���� | Na2CO3���� | / | ���� |
B | SiO2 | Fe2O3 | ���� | ���� |
C | FeCl2��Һ | FeCl3��Һ | Cu | ���� |
D | Cl2 | HCl | ����̼������Һ | ϴ�� |
A.AB.BC.CD.D
����Ŀ����ͼ��ʵ�����Ʊ�����������һϵ�����ʵ���װ��(�г��豸�� A �оƾ�������)��
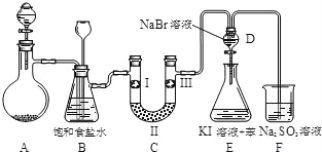
(1)ʵ���������������ӷ���ʽΪ_____��
(2)װ�� B �б���ʳ��ˮ��������_____����д��װ�� B ����һ������_______________________________��
(3)װ�� C ��ʵ��Ŀ������֤�����Ƿ����Ư���ԣ� Ϊ�� C ���������������η������ʵ������_______________(����)��
��� | a | b | c | d |
�� | �������ɫ���� | �������ɫ���� | ʪ�����ɫ���� | ʪ�����ɫ���� |
�� | ��ʯ�� | �轺 | Ũ���� | ��ˮ�Ȼ��� |
�� | ʪ�����ɫ���� | ʪ�����ɫ���� | �������ɫ���� | �������ɫ���� |
(4)���װ�� D��E ��Ŀ���DZȽ��ȡ��塢��ķǽ���������Ӧһ��ʱ���������װ�� D ��������Һ����װ�� E ���������������۲쵽��������_____����������˵����ķǽ�����ǿ�ڵ⣬ԭ����_________��
(5)װ�� F �������������ӷ�Ӧ����ʽ��_____��