题目内容
19. 铁、铜及其化合物在日常生产、生活有着广泛的应用.请回答下列问题:
铁、铜及其化合物在日常生产、生活有着广泛的应用.请回答下列问题:(1)铁在元素周期表中的位置第四周期第Ⅷ族.
(2)配合物Fe(CO)x常温下呈液态,熔点为-20.5℃,沸点为103℃,易溶于非极性溶剂,据此可判断Fe(CO)x晶体属于分子晶体(填晶体类型).Fe(CO)x的中心原子价电子数与配体提供电子数之和为18,则x=5.Fe(CO)x在一定条件下发生反应:Fe(CO)x(s)?Fe(s)+xCO(g).已知反应过程中只断裂配位键,由此判断该反应所形成的化学键类型为金属键.
(3)写出CO的一种常见等电子体分子的结构式N≡N;两者相比较沸点较高的为CO(填化学式).CN-中碳原子杂化轨道类型为sp杂化,S、N、O三元素的第一电离能最大的为N(用元素符号表示).
(4)铜晶体中铜原子的堆积方式如图1所示.
①基态铜原子的核外电子排布式为[Ar]3d104s1 或1s22s22p63s23p63d104s1.
②每个铜原子周围距离最近的铜原子数目12.
(5)某M原子的外围电子排布式为3s23p5,铜与M形成化合物的晶胞如附图2所示(黑点代表铜原子).
①该晶体的化学式为CuCl.
②已知铜和M的电负性分别为1.9和3.0,则铜与M形成的化合物属于共价(填“离子”、“共价”)化合物.
③已知该晶体的密度为ρg.cm-3,阿伏伽德罗常数为NA,则该晶体中铜原子和M原子之间的最短距离为\frac{\sqrt{3}}{4}\root{3}{\frac{4×99.5}{ρ{N}_{A}}}×1010pm(只写计算式).
分析 (1)铁是26号元素,位于周期表中第四周期第ⅤⅢ族;
(2)分子晶体的熔沸点较低;配合物Fe(CO)x的中心原子是铁原子,其价电子数是8,每个配体提供的电子数是2,据此判断x值;根据生成物判断形成的化学键;
(3)原子数和价电子数相同的微粒互为等电子体;根据分子的极性判断,极性分子的沸点较高;根据C原子价层电子对个数确定杂化方式;一般来说非金属性越强,第一电离能越大,但是因为p轨道半充满体系具有很强的稳定性,会有特例,如N的p轨道本来就是半充满的,O的p轨道失去一个电子才是半充满的,所以O比N容易失去电子;
(4)①铜为29号元素,据此写出基态铜原子的核外电子排布式;
②根据晶胞结构图可知,铜为面心立方堆积,据此判断每个铜原子周围距离最近的铜原子数目;
(5)根据价电子排布式判断出X原子为Cl原子;
①利用均摊法计算得出;
②根据电负性差值判断化合物类型;
③计算出一个晶胞中微粒数,利用化合物的摩尔质量和密度计算出晶胞边长,根据晶胞的结构可知,铜原子和M原子之间的最短距离为立方体体对角线的14.
解答 解:(1)铁是26号元素,位于周期表中第四周期第ⅤⅢ族,
故答案为:第四周期第ⅤⅢ族;
(2)分子晶体的熔沸点较低,根据题给信息知,该物质的熔沸点较低,所以为分子晶体,配合物Fe(CO)x的中心原子是铁原子,其价电子数是8,每个配体提供的电子数是2,8+2x=18,x=5,Fe(CO)5在一定条件下发生分解反应:Fe(CO)5=Fe(s)+5CO,反应生成Fe,所以形成的化学键为金属键,
故答案为:分子晶体;5;金属键;
(3)原子数和价电子数相同的微粒互为等电子体,则CO的一种常见等电子体分子的结构式为N≡N;根据分子的极性判断,极性分子的沸点较高,CO为极性分子,所以CO的沸点较高;CN-中C原子价层电子对个数=1+12(4+1-1×3)=2,所以采取sp杂化;一般来说非金属性越强,第一电离能大,但是因为p轨道半充满体系具有很强的稳定性.N的p轨道本来就是半充满的.O的p轨道失去一个电子才是半充满的.所以C、N、O三元素的第一电离能由大到小的顺序为N>O>C,即N的第一电离能最大,
故答案为:N≡N;CO;sp杂化;N;
(4)①铜为29号元素,基态铜原子的核外电子排布式为[Ar]3d104s1 或1s22s22p63s23p63d104s1,
故答案为:[Ar]3d104s1 或1s22s22p63s23p63d104s1;
②根据晶胞结构图可知,铜为面心立方堆积,所以每个铜原子周围距离最近的铜原子位于经过该原子的立方体的面的面心上,共有12个,
故答案为:12;
(5)根据价电子排布式判断出X原子为Cl原子;
①由晶胞结构可知,Cu原子处于晶胞内部,晶胞中含有4个Cu原子,Cl原子属于顶点与面心上,晶胞中含有Cl原子数目为8×18+6×12=4,故化学式为CuCl,
故答案为:CuCl;
②电负性差值大于1.7原子间易形成离子键,小于1.7原子间形成共价键,铜与X的电负性分别为1.9和3.0,差值为1.1小于1.7,形成共价键,
故答案为:共价;
③一个晶胞的摩尔质量为4×99.5g/mol,晶胞摩尔体积为4×99.5ρcm3,晶胞的边长为\root{3}{\frac{4×99.5}{ρ{N}_{A}}}cm,根据晶胞的结构可知,铜原子和M原子之间的最短距离为立方体体对角线的14,而体对角线为晶胞边长的√3倍,所以铜原子和M原子之间的最短距离为√34×\root{3}{\frac{4×99.5}{ρ{N}_{A}}}cm=\frac{\sqrt{3}}{4}\root{3}{\frac{4×99.5}{ρ{N}_{A}}}×1010pm,
故答案为:\frac{\sqrt{3}}{4}\root{3}{\frac{4×99.5}{ρ{N}_{A}}}×1010.
点评 本题考查较为全面,涉及到电子排布式、第一电离能、杂化类型的判断、配合物以及有关晶体的计算,但解题具有较强的方法性和规律性,学习中注意总结书写电子排布式的方法,如何判断分子空间构型以及有关晶体计算等方法.

 阅读快车系列答案
阅读快车系列答案
| A. | 铜电极上发生还原反应 | |
| B. | 电池工作时,铜电极附近会出现蓝色 | |
| C. | 锌片失去的电子通过番茄汁流向铜电极 | |
| D. | 工作一段时间后,两极质量均减轻 |
 (一)实验方法测定反应热---------中和热测定
(一)实验方法测定反应热---------中和热测定(1)实验桌上备有烧杯(大、小两个烧杯)、泡沫塑料、泡沫塑料板、胶头滴管、环形玻璃棒、0.5mol•L-1 盐酸、0.55mol•L-1NaOH溶液,尚缺少的实验玻璃用品是量筒、温度计.
| 实 验 用 品 | 溶 液 温 度 | 中和热△H | |||
| t1 | t2 | ||||
| ① | 50mL0.55mol.L-1NaOH | 50mL.0.5mol.L-1Cl | 20℃ | 23.3℃ | |
| ② | 50mL0.55mol.L-1NaOH | 50mL.0.5mol.L-1HCl | 20℃ | 23.5℃ | |
已知:Q=Cm(t2-t1),反应后溶液的比热容C为4.18KJ•℃-1•Kg-1,各物质的密度均为1g•cm-3.计算完成表格.△H=-56.8kJ/mol
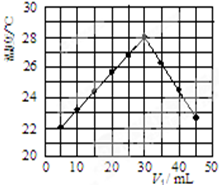
(3)某研究小组将V1 mL 1.0mol/L HCl溶液和V2 mL未知浓度的NaOH溶液混合均匀后测量并记录溶液温度,实验结果如图所示(实验中始终保持V1+V2=50mL).回答下列问题:
研究小组做该实验时环境温度低于(填“高于”、“低于”或“等于”)22℃,此反应所用NaOH溶液的浓度应为1.5mol/L.
(二)通过化学计算间接获得
(1)已知拆开1mol的H-H键、I-I、H-I键分别需要吸收的能量为436kJ、153kJ、299kJ.则反应H2(g)+I2(g)=2HI(g)的反应热△H=-9 kJ•mol-1
(2)已知:2H2(g)+O2(g)=2H2O (l)△H=-571.6kJ•mol-1H2(g)+1/2O2(g)=H2O(g)△H=-241.8kJ•mol-1
根据上述反应确定:H2燃烧热为285.8kJ•mol-1.
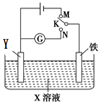 利用如图所示装置进行下列实验,下表中对应叙述正确的是( )
利用如图所示装置进行下列实验,下表中对应叙述正确的是( )| A | X为硫酸氢钠,Y为石墨 K与M连接时;K与N连接时 | 一段时间后溶液的pH均增大 |
| B | X为氯化钠,Y为石墨 K与M连接时; K与N连接时 | 石墨电极反应均为: 4OH--4e-═2H2O+O2↑ |
| C | X为硫酸氢钠,Y为锌 K与M连接时; K与N连接时 | 铁电极反应均为: 2H++2e-═H2↑ |
| D | X为氯化钠,Y为锌 K与M连接时; K与N连接时 | 铁电极的保护方法均为: 牺牲阳极的阴极保护法 |
| A. | A | B. | B | C. | C | D. | D |
| A. | 常温下1L 0.1 mol•L-1 NH4NO3溶液中的氮原子数为0.2NA | |
| B. | 含1mol H2SO4的浓硫酸和足量的锌完全反应,转移的电子数为2NA | |
| C. | 标准状况下2.24L己烷分子中含有1.9NA对共用电子 | |
| D. | 以Mg、Al为电极,NaOH溶液为电解质溶液的原电池中,导线上流过NA个电子,则正极放出H2的体积为11.2L |
 短周期元素W、X、Y、Z在元素周期表中的相对位置如表所示,这四种元素的原子最外层电子数之和为20.则下列说法不正确的是( )
短周期元素W、X、Y、Z在元素周期表中的相对位置如表所示,这四种元素的原子最外层电子数之和为20.则下列说法不正确的是( )| A. | 最高价氧化物的水化物的酸性:X<Z | |
| B. | 原子半径大小:Y<X<W | |
| C. | 氢化物的稳定性X<Y | |
| D. | X和Y形成的化合物升华破坏的是共价键 |
| 主族 周期 | ⅠA | ⅡA | ⅢA | ⅣA | ⅤA | ⅥA | ⅦA | 0 |
| 2 | E | F | ||||||
| 3 | A | C | D | G | R | |||
| 4 | B | H |
(2)D元素的最高价氧化物对应的水化物与A元素的最高价氧化物对应的水化物反应的离子方程式是Al(OH)3+OH-=AlO2-+2H2O;
(3)A、B、C三种元素按原子半径由大到小的顺序排列为(写元素符号)K>Na>Mg;
(4)F元素氢化物的化学式是H2O,该氢化物含有的化学键是共价键,该氢化物在常温下跟B元素的单质发生反应的化学方程式是2K+2H2O=2KOH+H2↑;该氢化物的沸点比同主族其它元素的氢化物的沸点高(填“高”或“低”),原因是水中存在氢键.
(5)H元素跟A元素形成化合物的化学式是NaBr,高温灼烧该化合物时,火焰呈黄色;用电子式表示该化合物的形成过程
 .
.(6)G元素和H元素两者核电荷数之差是18.G和H的最高价氧化物对应的水化物的酸性较弱的是(写化学式)HBrO4.
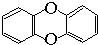 ,其一氯代物有( )
,其一氯代物有( )