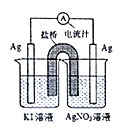
��Ŀ����
����Ŀ����.ijѧ���� 0.100 mol��L-1 �� KOH ����Һ�ζ�δ֪Ũ�ȵ����ᣬ ������ɷֽ�Ϊ���¼�����
A.��ȡ 20.00 mL ����������Һע��ྻ����ƿ�������� 2��3 �η�̪��
B. �� �� �� Һ �� ϴ �� �� �� 2��3�� ��
C.��ʢ�б���Һ�ļ�ʽ�ζ��̶ܹ��ã����ڵζ��ܼ���ʹ֮������Һ��
D.ȡ�� KOH ��Һע���ʽ�ζ������̶���0������ 2��3 mL�� E.����Һ������0������0�����¿̶ȣ����¶�����
F.����ƿ���ڵζ��ܵ����棬�ñ� KOH ��Һ�ζ����յ㲢���µζ���Һ��Ŀ̶ȡ�
�ʹ�ʵ�������գ�
(1)��ȷ���������˳����(����ĸ�����д)_____��
(2)���� A �������֮ǰ�������ô�����Һ��ϴ��ƿ����ζ����_________(����ƫ������ƫ��������������)��
(3)�ζ���(װ����Һ)�ڵζ�ǰ���촦�����ݣ��ζ�������������ʧ��ʹ�ζ����_____(����ƫ������ƫ��������������)��
��.��Ѫ�Ƶĺ���ʱ���ɽ� 2.0 mL ѪҺ������ˮϡ�ͺ��������м������������(NH4)2C2O4 ���壬��Ӧ���� CaC2O4 ��������������ϡ���ᴦ���� H2C2O4 ������������ KMnO4 ��Һ�ζ�����������Ϊ CO2����ԭ����Ϊ Mn2+�����յ�ʱ��ȥ 20.00 mL 1.0��10-4 mol��L-1 �� KMnO4 ��Һ��
(1)д���� KMnO4 �ζ� H2C2O4 �����ӷ���ʽ_____________��
(2)�жϵζ��յ�ķ�����_________��
(3)���㣺ѪҺ�к������ӵ�Ũ��Ϊ_____g��mL-1��
���𰸡�B��D��C��E��A��F ƫ�� ƫ�� 2MnO4-+5H2C2O4+6H+=2Mn2++10CO2��+8H2O ���������һ�����Ը��������Һ����Һ����ɫ��Ϊdz��ɫ���Ұ�����ڲ���ɫ 1.0��10-4
��������
��.��1���к͵ζ��м�©��ϴ�ӡ���ϴ��װҺ��ȡ����Һ����ƿ��Ȼ�����ָʾ�����еζ��Ȳ�����
��2��һԪǿ����һԪǿ�Ӧ��ȫ�к���������ʵ�����ȣ���c(��)V(��) = c(��) V(��)������V(��)�Ķ����жϲ��������������������Ӱ�죻
��3������Һ��ɫ�仯�Ұ�����ڲ���ɫ����˵���ﵽ�ζ��յ㣻
��.��1��������ؾ���ǿ�����ԣ������������½�H2C2O4����ΪCO2����������ԭΪMnSO4���ݴ�д����Ӧ�����ӷ���ʽ��
��2�����������Һ��������ɫ��Ϊ��ɫ���ڿ�ʼ���������ʱ����ԭ����ɫ��ʧ�����ﵽ�ζ��յ�ʱ���������һ�θ��������Һ��ɫ����ȥ����ҺӦ��������ɫ��Ϊdz��ɫ��
��3�������йط�Ӧ�ķ���ʽ���Եó���ϵʽ��5Ca2+��2KMnO4���ݴ˼�����
��1�������IJ�����ѡ��ζ��ܣ�Ȼ��ϴ�ӡ�װҺ��ʹ���������Һ���̶��ڵζ�̨�ϣ�Ȼ�����Һ����¶�������ȡ����Һ����ƿ��Ȼ�����ָʾ�����еζ�������˳��Ϊ��B��D��C��E��A��F��
�ʴ�Ϊ��B��D��C��E��A��F��
��2����ƿ������ˮϴ�Ӻ�������ô���Һ��ϴ����ʹ��ƿ�����ʵ����ʵ����������V(��)ƫ����c(��) = ![]() �жϻ���ɽ��ƫ����
�жϻ���ɽ��ƫ����
�ʴ�Ϊ��ƫ�ߣ�
��3���ζ���(װ����Һ)�ڵζ�ǰ���촦�����ݣ��ζ�������������ʧ����V(��)������ƫ��������c(��) = ![]() �жϻ���ɽ��ƫ����
�жϻ���ɽ��ƫ����
�ʴ�Ϊ��ƫ�ߣ�
��.��1��������ؾ���ǿ�����ԣ������������½�H2C2O4����ΪCO2����������ԭΪMnSO4����Ӧ����ʽΪ2KMnO4+5H2C2O4+3H2SO4�T2MnSO4+K2SO4+10CO2��+8H2O�������ӷ���ʽΪ��2MnO4-+5H2C2O4+6H+=2Mn2++10CO2��+8H2O��
�ʴ�Ϊ��2MnO4-+5H2C2O4+6H+=2Mn2++10CO2��+8H2O��
��2�����������Һ��������ɫ��Ϊ��ɫ���ڿ�ʼ���������ʱ����ԭ����ɫ��ʧ�����ﵽ�ζ��յ�ʱ���������һ�θ��������Һ��ɫ����ȥ����Һ����ɫ��Ϊdz��ɫ���Ұ�����ڲ���ɫ��
�ʴ�Ϊ�����������һ�����Ը��������Һ����Һ����ɫ��Ϊdz��ɫ���Ұ�����ڲ���ɫ��
��3����CaC2O4+H2SO4�TCaSO4+H2C2O4��2MnO4-+5H2C2O4+6H+=2Mn2++10CO2��+8H2O���Եó���ϵʽ��5Ca2+��2KMnO4������n(Ca2+)=2.5 n(KMnO4) = 1.0��10-4 mol/L��0.02L��2.5�����Կɼ����2 mL��ѪҺ�к��Ƶ����ʵ���Ϊ��1.0��10-4mol/L��0.02L��2.5 = 5��10-6 mol������n = ![]() �ɵã�m(Ca2+) = 5��10-6 mol��40 g/mol = 2��10-4 g������ѪҺ�к������ӵ�Ũ��Ϊ
�ɵã�m(Ca2+) = 5��10-6 mol��40 g/mol = 2��10-4 g������ѪҺ�к������ӵ�Ũ��Ϊ![]() = 1.0��10-4 g��mL-1��
= 1.0��10-4 g��mL-1��
�ʴ�Ϊ��1.0��10-4��

 ���Ӣ��������ϵ�д�
���Ӣ��������ϵ�д�����Ŀ����֪��ѧ��Ӧ��:Fe(s)+CO2(g)![]() FeO(s)+CO(g),�仯ѧƽ�ⳣ��ΪK1;��ѧ��Ӧ��:Fe(s)+H2O(g)
FeO(s)+CO(g),�仯ѧƽ�ⳣ��ΪK1;��ѧ��Ӧ��:Fe(s)+H2O(g)![]() FeO(s)+H2(g),�仯ѧƽ�ⳣ��ΪK2,���¶�973 K��1173 K�������,K1��K2��ֵ�ֱ�����:
FeO(s)+H2(g),�仯ѧƽ�ⳣ��ΪK2,���¶�973 K��1173 K�������,K1��K2��ֵ�ֱ�����:
�¶� | K1 | K2 |
973 K | 1.47 | 2.38 |
1 173 K | 2.15 | 1.67 |
(1)ͨ�������е���ֵ�����ƶ�:��Ӧ����_______(��������������������)��Ӧ��
(2)���з�Ӧ��:CO2(g)+H2(g)![]() CO(g)+H2O(g),����д���÷�Ӧ��ƽ�ⳣ��K3�ı���ʽ:K3=______��
CO(g)+H2O(g),����д���÷�Ӧ��ƽ�ⳣ��K3�ı���ʽ:K3=______��
(3)���ݷ�Ӧ����ڿ��Ƶ���K1��K2��K3֮��Ĺ�ϵʽΪ__________,�ݴ˹�ϵʽ���ϱ�����,���ƶϳ���Ӧ����________(��������������������)��Ӧ��
(4)Ҫʹ��Ӧ����һ�������½�����ƽ��������Ӧ�����ƶ�,�ɲ�ȡ�Ĵ�ʩ��______ ��_____ (��д��ĸ���)��
A.��С��Ӧ�������ݻ� B.����Ӧ�������ݻ�
C.�����¶� D.ʹ�ú��ʵĴ���
E.�跨��Сƽ����ϵ�е�CO��Ũ��
(5)ͼ�ס��ҷֱ��ʾ��Ӧ����t1ʱ�̴ﵽƽ��,��t2ʱ����ı�ij�������������仯�����:

��ͼ����t2ʱ�̷����ı��������__________��
��ͼ����t2ʱ�̷����ı��������__________��