��Ŀ����
����Ŀ������Ҫ����500 mL 1 mol��L��1������Һ��
��1������ȡ��������Ϊ98%���ܶ�Ϊ1.84 g��cm��3��Ũ����________mL��
�����²������裺
�ٰ����õ�Ũ�������ձ�������ע������ˮ�У����ò����������ܽ⡣
�ڰѢ�������Һ��ȴ��С��ת��500mL����ƿ�С�
�ۼ���������ƿ�м�����ˮ��Һ���̶���1��2cm�������ý�ͷ�ι�С�ĵμ�����ˮ����Һ��Һ��ײ���̶������С�
������������ˮϴ���ձ��Ͳ�����2��3�Σ�ÿ��ϴ�ӵ�Һ�嶼С��ת������ƿ��������ҡ�ȡ�
�ݽ�����ƿ�����������ҡ�ȡ�
����д���пհף�
��2�������������ȷ˳��Ϊ______________(�����)��
��3����ʵ������õ��IJ���������10ml��Ͳ�����������ձ���_________��
��4���������ʹ������Һ��Ũ��ƫ�ߵ���_________
A.ijͬѧ�۲�Һ��������ͼ��ʾ

B.û�н��в�������ܣ�
C.������ˮʱ���������˿̶��ߣ�������һ����ˮ��
D.������ƿ��ת����Һʱ(���������)��Һ��������ƿ����
��5����ʵ������г���(4)��Dѡ�����Ӧ��δ�������________
���𰸡�2.7 �٢ڢܢۢ� 500mL����ƿ����ͷ�ι� A ��������
��������
��1������ϡ�������������������������ҪŨ����������
��2������һ�����ʵ���Ũ����Һ��һ�㲽�裺��������ȡ��ϡ�͡���ȴ����Һ��ϴ����������ҡ����װƿ��ǩ���ݴ�����
��3����������һ�����ʵ���һ�㲽��ѡ����Ҫ������
��4���������������ʵ����ʵ�������Һ�����Ӱ�죬����c=![]() ������������
������������
��5�������������ʵ��ʧ���������ȵģ���Ҫ�������ơ�
��1����98%��Ũ����(��=1.84g/cm3)����500mL 1molL1H2SO4��Һ�����ƹ�����������������䣬����ҪŨ��������Ϊx mL��
��1.84g/cm3��x mL��98%=1mol/L��0.5L��98g/mol����ã�x��2.7���ʴ�Ϊ��2.7��
��2�����Ƹ�ϡ����IJ���Ϊ����������ȡ��ϡ�͡���ȴ����Һ��ϴ����������ҡ�ȵȣ�������������ȷ˳��Ϊ���٢ڢܢۢݣ�
��3��������Һ�IJ������裺���ȼ������Ҫ�����ʵ�������Ȼ����ƽ������������ձ����ܽ⣬ͬʱ�ò��������裬����Һ��ȴ�����º��ò�����������Һ��500ml����ƿ��Ȼ��ϴ���ձ��Ͳ�����2��3�Σ���ϴ��ҺҲע������ƿ��Ȼ��������ƿ��עˮ����Һ����̶���1��2 cmʱ�����ý�ͷ�ι���μ��룬����Һ����̶������У�Ȼ��ҡ�ȡ�װƿ���ڴ˹������õ��������У���ƽ��ҩ�ס��ձ�����������500ml����ƿ����ͷ�ιܣ���ȱ�ٵ�������500mL����ƿ����ͷ�ιܣ�
��4��A. ͼ��Ϊ���ӿ̶���,������Һ���ƫС,����c=n/V��֪��ҺŨ��ƫ�ߣ�A����ȷ��
B. û�н��в�������ܣ����²���������ģ����ʵ�������ƫС����ҺŨ��ƫ�ͣ�B�����
C. ������ˮʱ���������˿̶ȣ�������Һ���ƫ����ҺŨ�Ȼ�ƫ�ͣ�C�����
D. ������ƿ��ת����Һʱ(ʵ�鲽���)������Һ�ε�������ƿ���棬�������ʵ����ʵ���ƫС����ҺŨ�Ȼ�ƫ�ͣ�D�����
��ΪA��
��5��������ƿ��ת����Һʱ(���������)��Һ��������ƿ���棬���ʼ�С������ʵ��ʧ���������ȣ������������ƣ��ʴ�Ϊ���������ơ�

����Ŀ���״�����Ҫ�Ļ���ԭ�ϣ����л��ϳ��о��й㷺Ӧ�á�
I��(1)�ü״���ȡ�װ��ķ�ӦΪ![]() ��H
��H
��֪�÷�Ӧ����ػ�ѧ���ļ����������£�
���ۼ� | C�DO | H�DO | N�DH | C�DN |
���ܣ�kJ��mol-1 | 351 | 463 | 393 | 293 |
��÷�Ӧ����H=__________kJ��mol-1
����һ�������£���2mol CO��6mol H2ͨ��2L�ܱ������з������·�Ӧ
����Ӧ��![]() ��H<0 ��
��H<0 ��
����Ӧ��![]() ��H<0 ��
��H<0 ��
��Ӧ��t minʱ���ﵽƽ��״̬��ƽ��ʱCH3OH�����������(CH3OH)��ͼ��ʾ��
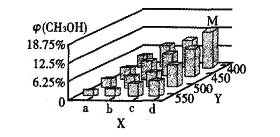
(2)ͼ��a___b(��������������С����)��ͼ��Y���ʾ�¶ȣ���������__________
(3)����ӦII��ƽ�ⳣ��Kֵ��С��������˵������ȷ����___________(�����)��
A��ƽ���������Ӧ�����ƶ�
B��ƽ���ƶ���ԭ�����������¶�
C���ﵽ��ƽ�����(CH3OH)��С
D����������(CH3OCH3)����
(4)ƽ��ʱ��M��CH3OH���������Ϊ12.5����c(CH3OCH3)=0.1mol��L-1�����ʱCO��ת����Ϊ________����H2��ʾI�ķ�Ӧ����Ϊ______mol��L-1��min-1����Ӧ����ƽ�ⳣ��K=___________(�÷�����ʾ)