��Ŀ����
11��ij��ѧ��ȤС���ij����李����ʽ�����ר���о�����1��Ԥ�⣺��������֪ʶ�͡���李������ƣ���С��Ԥ��á���李���̼�����������������Σ�
��2��ʵ���̽����
��NH4+����֤��ȡ��������ҩƷ���Թ��У�Ȼ��μ�NaOHŨ��Һ�������Թܣ���ʪ��ĺ�ɫʯ����ֽ�����Թܿڲ�������ɫʯ����ֽ��������֤����NH4+��
�������ӵ�̽����ȡ��������ҩƷ���Թ��У�Ȼ�����Թ��еμ�ϡ���ᣬ����ȫ���ܽ⣬������������ͨ��ͼ1װ�ã��۲쵽����������ˮ�����Ա仯������ʯ��ˮ����ǣ�����μ�ϡ���ᷴӦ����Թ��м�Ba��OH��2��Һ������������

�������ۣ�����李���̼�����Σ�
��˼��������ʵ�鲽��ڼ���Ba��OH��2��Һ��Ŀ���Ǽ����Ƿ����SO42-�����ݳ���ʵ����ۣ�������裺
����1����麟ɷ���NH4HCO3 ����2����麟ɷ��ǣ�NH4��2CO3
����3����麟ɷ��ǣ�NH4��2CO3��NH4HCO3�Ļ���
�ܸ����������裬���ʵ��ó����ۣ���ѡ�Լ��������У�CaCl2��Һ��Ʒ����Һ��Ba��NO3��2��Һ��1.0mol/L���ᡢ1.0mol•L-1 NaOH��Һ�����ͳ���ʯ��ˮ��BaCl2��Һ��Ba��OH��2��Һ���Թܡ���ͷ�ι�
| ʵ�鲽�� | Ԥ�������� |
| ����1��ȡ��������李����Ƴ���Һ��ȡ1��2ml��Һ��һ֧�ྻ���Թ��У�������BaCl2��Һ������� | �������ְ�ɫ������֤������к��У�NH4��2CO3�� ����û������������ܺ���NH4HCO3 |
| ����2�����ã�ȡ�ϲ���Һ��������һ�Թ��У� �����μӵμ�����Ba��OH��2��Һ���۲����� | �����ְ�ɫ������֤������к���NH4HCO3��ϲ���1�ڣ������1������ ��ϲ���1�٣������3������ ���ڲ���1�в�����ɫ�����������2�������� |
�ٸ�����麟ɷ֣����С���������ʵ��װ�����Ʊ�NH4HCO3�Ƿ��������ǡ���
�ڸ�ʵ��С�����������ʵ�鷽���ⶨ����еĺ������������Ķ���������ۣ�ȷ��ȡһ����������Ʒ������ͼ2��������ƿ�У��μӹ���NaOHŨ��Һ������ȣ�����ͼ3װ�ã�ͨ��ʵ��ⶨ�ձ����ӵ�����������©���и�����Һ����������Ȼ����м��㣬�ɵû��ʵĺ������������������������Ƿ������������ֻ��Ҫ˵�����ۣ��������������Ҫ˵��ԭ��ָ���ķ�������Ҫ����������ҩƷ����˵�����ӵ�λ�á����ƣ�
��������װ����û�и���װ�ã�ϡ���������˽϶�ˮ���������̫�����ձ�ǰ��һ��װ�м�ʯ�ҵĸ���ܣ�
���� ��2����笠����ӵļ��鷽���������������Ƽ��Ȳ�����ʹʪ��ĺ�ɫʯ����ֽ�����İ�����
����ʹʯ��ˮ����ǵ������Ƕ�����̼�����Ƕ����������壬�������ξ��л�ԭ�ԣ��ܱ���ˮ������̼���ο��Ժ��Ȼ�����Ӧ���ɳ������Ȼ�����̼�����β���Ӧ��
�۸������������ó�̼�����εIJ�ͬ�����
�ܸ���̼������ӡ�̼��������ӵ��ص������ʵ��ҩƷ��ѡ��ʵ������ķ�����
��3����ͼ2����������ˮ���ñ��Ͱ�ˮ����CO2��ȡ̼����泥�
����κ��������Ʒ�Ӧ���ɵİ����л���ˮ������Ű��������IJⶨ��
��� �⣺��2���ٸ���笠����ӵļ��鷽����������Ե�����м����������ƣ������ȣ��ܲ�����ʹʪ��ĺ�ɫʯ����ֽ�����İ�������֤����NH4+��
�ʴ�Ϊ���μ�NaOHŨ��Һ����ʪ��ĺ�ɫʯ����ֽ�����Թܿڲ��������Թܣ�����ɫʯ����ֽ��������֤����NH4+��
����ʹʯ��ˮ����ǵ������Ƕ�����̼�����Ƕ����������壬�������ξ��л�ԭ�ԣ��ܱ���ˮ��������ˮ�����Ա仯��˵������李���̼�����Σ�
�ʴ�Ϊ��̼�
�ۼ���Ba��OH��2��Һ��ַ�Ӧ������������ɫ������˵���û��ʵó���Һ����SO42-����������ϵ����淋ijɷֿ�����NH4HCO3��Ҳ�����ǣ�NH4��2CO3���������Ƕ��ߵĻ���
�ʴ�Ϊ�������Ƿ����SO42-������2����麟ɷ��ǣ�NH4��2CO3������3����麟ɷ��ǣ�NH4��2CO3��NH4HCO3�Ļ���
�ܸ����������裬���ʵ�飬����1��ȡ��������李����Ƴ���Һ��ȡ1��2ml��Һ��һ֧�ྻ���Թ��У�������BaCl2��Һ��ַ�Ӧ����������ɫ������˵����̼����泥�������̼��泥�����2�����ã�ȡ�ϲ���Һ��������һ�Թ��У������μ�����������������Һ�������ְ�ɫ������֤������к���NH4HCO3��ϲ���1�ڣ������1��������ϲ���1�٣������3��������û�а�ɫ���������������2����
�ʴ�Ϊ��
| ʵ�鲽�� | Ԥ�������� |
| ����1��ȡ��������李����Ƴ���Һ��ȡ1��2ml��Һ��һ֧�ྻ���Թ��У�������BaCl2��Һ������� | �������ְ�ɫ������֤������к��У�NH4��2CO3�� ����û������������ܺ���NH4HCO3 |
| ����2�����ã�ȡ�ϲ���Һ��������һ�Թ��У� �����μӵμ�����Ba��OH��2��Һ���۲����� | �����ְ�ɫ������֤������к���NH4HCO3��ϲ���1�ڣ������1������ ��ϲ���1�٣������3������ ���ڲ���1�в�����ɫ�����������2������ |
�ʴ�Ϊ����
����κ��������Ʒ�Ӧ���ɵİ����л���ˮ������Ű��������IJⶨ����Ҫ���ձ�ǰ��һ��װ�м�ʯ�ҵĸ���ܣ�
�ʴ�Ϊ����������װ����û�и���װ�ã�ϡ���������˽϶�ˮ���������̫�����ձ�ǰ��һ��װ�м�ʯ�ҵĸ���ܣ�
���� ������һ���йػ�ѧ���ϵ�������ɺ��жϵ���Ŀ������ѧ�������ͽ��������������ѶȽϴ�

| A�� | ��ԭ��X-ǿ��Y2- | |
| B�� | X�ĺ���������Ա�Y�ĺ����������ǿ | |
| C�� | X�ĵ���X2�ܽ�Y��������Y2-�������������û���Ӧ | |
| D�� | X���⻯���Y���⻯��е�� |
 ��Ӧ��L��s��+aG��g��?bR��g�� �ﵽƽ��ʱ���¶Ⱥ�ѹǿ�Ը÷�Ӧ��Ӱ����ͼ��ʾ��ͼ��ѹǿp1��p2��x���ʾ�¶ȣ�y���ʾƽ��������G�����������
��Ӧ��L��s��+aG��g��?bR��g�� �ﵽƽ��ʱ���¶Ⱥ�ѹǿ�Ը÷�Ӧ��Ӱ����ͼ��ʾ��ͼ��ѹǿp1��p2��x���ʾ�¶ȣ�y���ʾƽ��������G������������ݴ˿��жϣ�������
| A�� | a��b | B�� | ������Ӧ�����ȷ�Ӧ | ||
| C�� | a��b | D�� | ������Ӧ�Ƿ��ȷ�Ӧ |
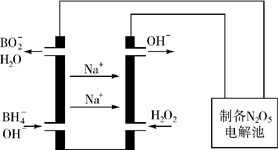 ������Ҫ�������簱���£�N2H4����NO��NO2��HNO3�������εȣ��������������о�����Ҫ���ã�
������Ҫ�������簱���£�N2H4����NO��NO2��HNO3�������εȣ��������������о�����Ҫ���ã���1������NH3�Ļ�ԭ�Կ����������������Ⱦ������Ȼ�ѧ����ʽ���£�
H2O��l��=H2O��g����H1=+44.0kJ•mol-1
N2��g��+O2��g��=2NO��g����H2=+229.3kJ•mol-1
4NH3��g��+5O2��g��=4NO��g��+6H2O��g����H3=-906.5kJ•mol-1
4NH3��g��+6NO��g��=5N2��g��+6H2O��l����H4�����H4=-2317.0kJ•mol-1��
��2����������һ�����ᣬ��֤����������������ʵ���AD
A�������£�����������Һ��pH��7
B���������ܺ�NaOH�����кͷ�Ӧ
C�������� �� ��Һ��������ʵ�飬���ݺܰ�
D�������£���pH=3����������Һϡ��10����pH��4
��3��һ���¶��£���a mol/L�İ�ˮ��b mol/LH2SO4��Һ�������ϣ���ַ�Ӧ���У�2c��SO42-��=c��NH4+�����ú�a��b�Ĵ���ʽ��ʾ�û����Һ��NH3•H2O�ĵ��볣��Ϊ$\frac{2b}{a-2b}$��10-7��
��4��ʹ��NaBH4Ϊ�յ�������ʹCo2+�����ڼ��������·�����Ӧ���Ƶøߴ��������ܣ��ù��̲������ж����壬д���÷�Ӧ�����ӷ���ʽ��2Co2++N2H4+4OH-=2Co��+N2��+4H2O��
��5��X��Y��Z��W�ֱ���HNO3��NH4NO3��NaOH��NaNO2����ǿ������е�һ�֣��±��dz�����Ũ�Ⱦ�Ϊ0.01mol•L-1��X��Y��Z��W��Һ��pH��
| 0.01mol•L-1����Һ | X | Y | Z | W |
| pH | 12 | 2 | 8.5 | 4.5 |

| A�� | ��������������Ӧ | B�� | �ձ��е���Һ��Ϊ��ɫ | ||
| C�� | ������ͭƬͨ����������пƬ | D�� | ��װ���ܽ�����ת��Ϊ��ѧ�� |

| A�� | 0��t2ʱ��v��ʼ�մ���v�� | |
| B�� | �������̴ﵽƽ��ʱ��A����������� | |
| C�� | �������̴ﵽƽ��ʱ��ƽ�ⳣ���� | |
| D�� | t2ʱ�̸ı���������������ܱ������м�C |
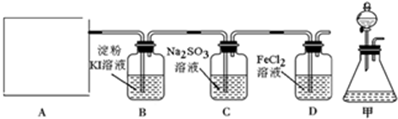

 ��
��