��Ŀ����
15������0.1mol•L-1��Na2SO4��0.1mol•L-1��H2SO4�����Һ100mL����������μ���0.2mol•L-1�� Ba��OH��2��Һ�������Ͻ��裬ʹ��Ӧ��ֽ��У������Ի�Ϲ����е�����仯����1��������50mLBa��OH��2��Һʱ��������Һ�е�������Na2SO4�������ʵ���Ũ��Ϊ0.067 mol•
L-1��
��2������Һ�г������ﵽ���ʱ������Ba��OH��2��Һ�����Ϊ100mL��������Һ������ΪNaOH������������ʵ���Ũ��Ϊ0.1mol•L-1��
���� ��1����Ӧ�൱��Ba��OH��2����H2SO4��Ӧ��Ȼ����Na2SO4�뷴Ӧ��50mLBa��OH��2��Һ��n[Ba��OH��2]=0.05L��0.2moL•L-1=0.01mol��100mL��Һ��n��H2SO4��=0.1L��0.1moL•L-1=0.01mol������������������ǡ�÷�Ӧ�������Ʋ���Ӧ������n=cV���������Ƶ����ʵ������ٸ���m=nM���������Ƶ�������
��2����Һ�г������ﵽ���ʱ������Ba2++SO42-=BaSO4������n[Ba��OH��2]=n��SO42-�����ٸ���V=$\frac{n}{V}$���������������������Һ������ΪNaOH�������������غ��֪n��NaOH��=n��Na+�����ٸ���c=$\frac{n}{V}$���㣮
��� �⣺��1����Ӧ�൱��Ba��OH��2����H2SO4��Ӧ��Ȼ����Na2SO4�뷴Ӧ��100mLBa��OH��2��Һ��n[Ba��OH��2]=0.1L��0.2moL•L-1=0.02mol��100mL��Һ��n��H2SO4��=0.1L��0.2moL•L-1=0.02mol������������������ǡ�÷�Ӧ�������Ʋ���Ӧ����Ӧ�����ӷ���ʽΪ��2H++SO42-+Ba2++2OH-=Ba SO4��+2 H2O����Һ������ΪNa2SO4��Na2SO4�����ʵ���Ϊ0.1L��0.1moL•L-1=0.01mol��Na2SO4��Ũ��=$\frac{0.01mol}{0.15L}$=0.067molL��
�ʴ�Ϊ��Na2SO4��0.067��
��2����Һ�г������ﵽ���ʱ���������ȫ��Ӧ������Ba2++SO42-=BaSO4������n[Ba��OH��2]=n��SO42-��=0.01mol+0.01mol=0.02mol��������������Һ�����Ϊ$\frac{0.02mol}{0.2mol/L}$=0.1L=100mL��
��ʱ��Һ������ΪNaOH�������������غ��֪n��NaOH��=n��Na+��=0.01mol��2=0.02mol������Һ��NaOHŨ��Ϊ=$\frac{0.02mol}{0.2L}$=0.1mol/L��
�ʴ�Ϊ��100��NaOH��0.1��
���� ���⿼��������йؼ��㡢���û�ѧ�������йؼ���ȣ��Ѷ��еȣ����ⷢ����Ӧ�ı��ʺ�Ԫ���غ��ǽ���Ĺؼ���

��ʽ̼���ܣ�Co4��OH��,��CO3��4�ݳ��������Ӳ��ϡ����Բ��ϵ����Ӽ���������ˮ������ʱ�ɷֽ��������������Ϊ��ȷ������ɣ�ij��ѧ��ȤС��ͬѧ�������ͼ��ʾ��װ�ã����������������顣
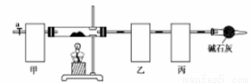
ʵ�鲽�����£�
�ٳ�ȡ3.65g��Ʒ����Ӳ�ʲ������ڣ������ҡ���װ�õ�������
�ڰ���ͼ��ʾװ����װ������������ ��
�ۼ���Ӳ�ʲ����ܣ�����װ���� ������ֹͣ���ȣ�
�ܴ���a������ͨ����������Ӻ����ҡ���װ�õ�������
�ݼ��㡣
��1��������ͼʾѡ��������װ�����ڷ����У�ʹ����ʵ��װ��������ѡ����ĸ��ţ����ظ�ѡ��
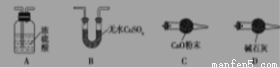
�ף� �ң� ����
��װ�õ������� ��
��2���������ʡ�Ե�ʵ�����Ϊ ��
���������װ�õ�����Ϊ ��
������л���ͨ����������ӵ�Ŀ���� ��
��3��������ȷװ�ý���ʵ�飬����������ݡ�
��װ�õ�����/g | ��װ�õ�����/g | |
����ǰ | 80.00 | 62.00 |
���Ⱥ� | 80.36 | 62.88 |
��ü�ʽ̼���ܵĻ�ѧʽΪ_____________��
��4��CO2��SO2��Ϊ�������壬�������ơ�Ϊ�˱Ƚ��������̼�������ǿ����ijͬѧ������װ�ý���ʵ�顣
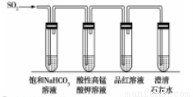
��д����ʵ���ܴﵽʵ��Ŀ�ĵ�ʵ������____________��
������SO2ͨ��ˮ�������ͣ������ʵ��֤�������������ᣬʵ�鷽��Ϊ____________��
| A�� | 2��3 | B�� | 3��1 | C�� | 2��1 | D�� | 1��2 |
| A�� | ����Ӧ��Ũ�ȿɼӿ췴Ӧ���ʣ������Ũ����������Ӧ����������H2������ | |
| B�� | NH4Fˮ��Һ�к���HF�����NH4F��Һ���ܴ���ڲ����Լ�ƿ�� | |
| C�� | ��ȼ����Ҫ�Ǽ�����ˮ�ڵ��¸�ѹ���γɵ�ˮ���ᄃ�壬��˿ɴ����ں��� | |
| D�� | ij���ȷ�Ӧ���Է����У���˸÷�Ӧ��������Ӧ |
| ���� | HF | H2CO3 | HClO |
| ����ƽ�ⳣ����Ka�� | 7.2��10-4 | Kal=4.4��10-7 Ka2=4.7��10-11 | 3.0��10-8 |
mol-1����H+��aq��+OH-��aq���TH2O��l����H=-57.3kJ•mol-1
�����ĵ��뷽��ʽ����ЧӦ�ɱ�ʾΪHF��aq��?H+��aq��+F-��aq����H=-10.4KJ•mol-1��
��2����Ũ��Ϊ0.1mol•L-1��HF��Һ��ˮϡ��һ���������¶Ȳ��䣩�����и����е�ֵ���������CD��
A��c��H+�� B��c��H+��•c��OH-�� C.$\frac{c��H+��}{c��HF��}$ D.$\frac{c��OH-��}{c��H+��}$
��3�����з����У�����ʹ0.10mol•L-1 HF��Һ��HF����̶��������d����������ĸ��ע�����ִ�Сд����Сд������÷֣�
a�������¶�
b������Һ�е���2��Ũ����
c����������NaF����
d����ˮϡ�ͣ�
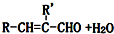

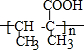 ��
��