��Ŀ����
����Ŀ��ˮú���任[CO(g)+H2O(g)![]() CO2(g)+H2(g)]����Ҫ�Ļ������̣���Ҫ���ںϳɰ��������Լ��ϳ����ӹ��ȹ�ҵ�����С��ش��������⣺
CO2(g)+H2(g)]����Ҫ�Ļ������̣���Ҫ���ںϳɰ��������Լ��ϳ����ӹ��ȹ�ҵ�����С��ش��������⣺
��1���ҹ�ѧ�߽��ʵ��������ģ�������о����ڽ����������ˮú���任�ķ�Ӧ���̣���ͼ��ʾ�����������ڽ���������ϵ�������![]() ��ע��
��ע��

�������о��ٲ���Ļ�ѧ����ʽΪ__������ʽ����������ͬ���ϲ���Լ��ˮú���任��Ӧ���Ȼ�ѧ����ʽΪ__��
��2��t1��ʱ���ܱ������У�ͨ��һ������CO��H2O������ˮú���任��Ӧ�������и�����Ũ��(��λ��mol��L-1)�仯���±���ʾ��
ʱ��(min) | CO | H2O | CO2 | H2 |
0 | 0.200 | 0.300 | 0 | 0 |
2 | 0.138 | 0.238 | 0.062 | 0.062 |
3 | c1 | c2 | c3 | c3 |
4 | c1 | c2 | c3 | c3 |
5 | 0.116 | 0.216 | 0.084 | |
6 | 0.096 | 0.266 | 0.104 |
��һ������ƽ��״̬��ʱ���Ϊ__��
��5��6minʱ����ڣ�ƽ���ƶ�����Ϊ__(������ƶ����������ƶ���)�����ݱ��������жϣ�ƽ���ƶ���ԭ����__(����ĸ���)��
a.������H2O(g)���� b.��������Ũ�� c.ʹ�ô��� d.�����¶�
��t2��ʱ(t2��t1)������ͬ�����·���������Ӧ����ƽ��ʱ��COŨ��__c1(�������������=��)��
��3����֪��ӦFe(s)+CO2(g)![]() FeO(s)+CO(g)��ƽ�ⳣ�����¶ȱ仯�����ͼ��ʾ��
FeO(s)+CO(g)��ƽ�ⳣ�����¶ȱ仯�����ͼ��ʾ��

�ٸ÷�Ӧ��__(����ȡ����ȡ�)��Ӧ��
����T1ʱˮú���任��Ӧ��ƽ�ⳣ������0.5����T1ʱFeO(s)+H2(g)![]() Fe(s)+H2O(g)��ƽ�ⳣ��Ϊ__��
Fe(s)+H2O(g)��ƽ�ⳣ��Ϊ__��
��4��ˮú������ȼ�ϵ�ص�ȼ�ϡ�һ������̼����ȼ�ϵ�صĹ���ԭ����ͼ��ʾ���缫A��H2����ĵ缫��ӦΪ__�������¯������CO��H2���ʵ���֮��Ϊ1�U2���缫A��������CO2�в��ֲ���ѭ�����ã���������Ϊ__��

���𰸡�COOH![]() +H
+H![]() +H2O
+H2O![]() =COOH
=COOH![]() +2H
+2H![]() +OH
+OH![]() CO(g)+H2O(g)
CO(g)+H2O(g)![]() CO2(g)+H2(g)
CO2(g)+H2(g) ![]() H=-0.72NAeV/mol 3min��4min �����ƶ� a �� ���� 1 H2+CO32--2e-=H2O+CO2 75%
H=-0.72NAeV/mol 3min��4min �����ƶ� a �� ���� 1 H2+CO32--2e-=H2O+CO2 75%
��������
(1)��ʼ״̬��Ӧ����������Ϊ0������״̬��������������Ϊ-0.72eV����Ӧ������������������������ˮú���任��ӦΪ���ȷ�Ӧ������HС��0�����������������ݷֱ�Ϊ1.59eV-(-0.32eV)=1.91eV��1.86eV-(-0.16eV)=2.02eV���������(���)E��=2.02eV���ò���ķ�Ӧ����������Ϊ-0.16eV�����ʣ�COOH![]() +H
+H![]() +H2O
+H2O![]() ��������Ϊ�������Ϊ1.41eV�����ʣ�COOH
��������Ϊ�������Ϊ1.41eV�����ʣ�COOH![]() +2H
+2H![]() +OH
+OH![]() ���ɴ˿ɵøò���Ļ�ѧ����ʽ��COOH
���ɴ˿ɵøò���Ļ�ѧ����ʽ��COOH![]() +H
+H![]() +H2O
+H2O![]() =COOH
=COOH![]() +2H
+2H![]() +OH
+OH![]() ��ˮ���任��Ӧ���Ȼ�ѧ����ʽΪCO(g)+H2O(g)
��ˮ���任��Ӧ���Ȼ�ѧ����ʽΪCO(g)+H2O(g)![]() CO2(g)+H2(g)
CO2(g)+H2(g) ![]() H=-0.72NAeV/mol��
H=-0.72NAeV/mol��
(2)�ٴӱ��������ݷ�������3��4minʱ����ϵ�и����ʵ�Ũ�Ȳ��ٱ仯��˵���Ѿ��ﵽƽ��״̬��
��5min��6minʱ����ڣ�H2O��Ũ������COŨ�ȼ�С��˵����������H2O������ƽ�����ƣ��ʴ�Ϊ�������ƶ���a��
�۸÷�Ӧ������ӦΪ���ȷ�Ӧ�������¶�ƽ�������ƶ���COת���ʼ�С����COŨ�ȣ�c1,�ʴ�Ϊ������
(3)�������¶Ȼ�ѧƽ�ⳣ������˵��Fe(s)+CO2(g)![]() FeO(s)+CO(g)Ϊ���ȷ�Ӧ���ʴ�Ϊ�����ȣ�
FeO(s)+CO(g)Ϊ���ȷ�Ӧ���ʴ�Ϊ�����ȣ�
��T1ʱ��ӦFe(s)+CO2(g)![]() FeO(s)+CO(g)��ƽ�ⳣ��K=2����c(CO)/c(CO2)=2����T1ʱˮú���任��Ӧ��ƽ�ⳣ������0.5����K=
FeO(s)+CO(g)��ƽ�ⳣ��K=2����c(CO)/c(CO2)=2����T1ʱˮú���任��Ӧ��ƽ�ⳣ������0.5����K=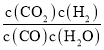 ��0.5������ƽ��ʱ
��0.5������ƽ��ʱ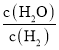 ��1������T1ʱFeO(s)+H2(g)
��1������T1ʱFeO(s)+H2(g)![]() Fe(s)+H2O(g)��ƽ�ⳣ��K=
Fe(s)+H2O(g)��ƽ�ⳣ��K=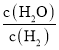 ��1��
��1��
(4)AΪ������������CO��H2���������ɶ�����̼��ˮ����缫A��ǰȥ����ķ�ӦΪ��H2+CO32--2e-=H2O+CO2�������¯������CO��H2���ʵ����ֱ�Ϊ1mol��2mol���缫A��ת�Ƶ���Ϊ6mol��������CO2Ϊ4mol�������������ĵ缫��ӦΪO2+2CO2+4e-=2CO32-����ת��6mol����ʱ������ѭ����Ӧ��CO2Ϊ3mol������ѭ����������=![]() =75
=75![]() ��
��

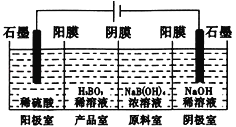
����Ŀ��ij����С����Ƶ�ʵ������ȡ����������װ����ͼ��ʾ����֪����ˮ�Ȼ��ƿ����Ҵ��γ�������ˮ��CaCl2��6C2H5OH�����й��л�����ķе����±���
�Լ� | ���� | �Ҵ� | ���� | �������� |
�е㣨�棩 | 34.7 | 78.5 | 118 | 77.1 |
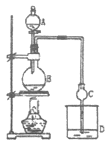
A�зŴ��ᣬB�н��������Ҵ�������Ũ�����ϣ�D�з��б���̼������Һ������Һ©���ߵμӴ��ᡢ���ȡ�
��ش�
��1����Ӧ���������ƣ�Բ������ƿ���ȼ���___������μ���___���ӱ���___���ټ�����ᡣ
��2����Ӧ�м�����Ҵ��ǹ����ģ���Ŀ����___��Ũ��������ã���___����___��
��3���÷�Ӧ�У�����CH3CH218OH�����ᷢ����Ӧ����Ӧ�Ļ�ѧ����ʽΪ___��
��4�����ȳ��˼ӿ췴Ӧ�������һ����Ҫ��Ŀ�ģ�___��
��5����װ���У����θ������Ҫ���������ã�һ�����������ã����ǣ�___��
��6���Թ���ʢ�ŵ��DZ���̼������Һ������������___(ѡ����)��
A.�к�������Ҵ� B.�������Ტ�ܽ��Ҵ�
C.���������������ܽ�ȣ����������� D.�����������������ɣ���������
��7����D�з���������������г�����һ�������Ҵ������Ѻ�ˮ��Ӧ�ȼ�����ˮ�Ȼ��ƣ������___���ټ���___(�˿մ�����ѡ����ѡ��)��Ŀ����___��Ȼ����������ռ�77�����ҵ���֣��Եõ��ϴ���������������
A.��ʯ�� B.��ˮ������ C.��ʯ��
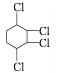
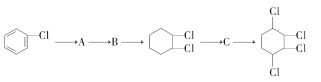
����Ŀ���ڸ��������£���������������ӹ����жϼ���Ӧ�����ӷ���ʽ����ȷ����
ѡ�� | ���� | ������ | ���ӹ����жϼ������ӷ���ʽ |
A |
| Fe2+��NO3-��Al3+��Cl- | ���ܴ��������� 3Fe2++4H++NO3-=3Fe3++NO+2H2O |
B | ��ˮ�����c(H+) =1��10-13mol��L-1 | K+��NH4+��Cl-��AlO2- | �ܴ������� |
C | ���д���Al3+����Һ | Na+��NH4+��SO42-��Cl- | �ܴ������� |
D | ͨ������SO2���� | K+��Na+��ClO-��SO42- | ���ܴ������棬 2ClO-+SO2+H2O��2HClO+SO32- |
A. A B. B C. C D. D