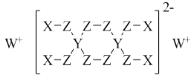��Ŀ����
����Ŀ�������� YX2��ZX2 �У�X��Y��Z �ĺ˵����С�� 18��X ԭ�������ܲ�� p �ܼ�����һ����������� 2 �����ӣ�Y ԭ�ӵ�������� p �ܼ��ĵ���������ǰһ�ܲ������������X �� Y ������ͬ�ĵ��Ӳ�����Z �� X �����ڱ���λ��ͬһ���塣�ش��������⣺
��1��X �ĵ����Ų�ʽΪ______________��Y �ļ۵��ӹ����ʾʽΪ_______��
��2��ZX2 �ķ���ʽ��_______��YX2 ����ʽ��_______ ��
��3��Z �γɵļ������ӵĽṹʾ��ͼ��___________��
��4�� Y �� Z һ���������γɼ��ӵĽṹʽ��___________________��
���𰸡�1s22s22p4 ![]() SO2
SO2 ![]()
 S��C��S
S��C��S
��������
������YX2��ZX2�У�X��Y��Z�ĺ˵����С��18��Xԭ�������ܲ��p�ܼ�����һ�����������2�����ӣ�����X������Yԭ�ӵ��������p�ܼ��ĵ���������ǰһ�ܲ�������������Ӧ��Ϊ2����X��Y������ͬ�ĵ��Ӳ㣬����Y��̼��Z��X�����ڱ���λ��ͬһ���壬����Z���ݴ˽��
�������Ϸ�����֪X��O��Y��C��Z��S����
��1��X��O��X�ĵ����Ų�ʽΪ1s22s22p4��Y��̼Ԫ�أ���Y�Ĺ����ʾʽΪ![]() ��
��
�ʴ�Ϊ��1s22s22p4 �� ![]() ��
��
��2��ZX2�ķ���ʽ��SO2��YX2�ķ���ʽ��CO2������ʽ��![]() ��
��
�ʴ�Ϊ��SO2 ��![]() ��
��
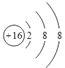
��3��Z�γɵļ���������S2-����ṹʾ��ͼ��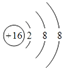 ��
��
�ʴ�Ϊ�� 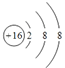 ��
��
��4��Y��Z��һ���������γɼ�����CS2����ṹʽ��S��C��S��
�ʴ�Ϊ�� S��C��S��

����Ŀ��ˮú���任[CO(g)+H2O(g)![]() CO2(g)+H2(g)]����Ҫ�Ļ������̣���Ҫ���ںϳɰ��������Լ��ϳ����ӹ��ȹ�ҵ�����С��ش��������⣺
CO2(g)+H2(g)]����Ҫ�Ļ������̣���Ҫ���ںϳɰ��������Լ��ϳ����ӹ��ȹ�ҵ�����С��ش��������⣺
��1���ҹ�ѧ�߽��ʵ��������ģ�������о����ڽ����������ˮú���任�ķ�Ӧ���̣���ͼ��ʾ�����������ڽ���������ϵ�������![]() ��ע��
��ע��

�������о��ٲ���Ļ�ѧ����ʽΪ__������ʽ����������ͬ���ϲ���Լ��ˮú���任��Ӧ���Ȼ�ѧ����ʽΪ__��
��2��t1��ʱ���ܱ������У�ͨ��һ������CO��H2O������ˮú���任��Ӧ�������и�����Ũ��(��λ��mol��L-1)�仯���±���ʾ��
ʱ��(min) | CO | H2O | CO2 | H2 |
0 | 0.200 | 0.300 | 0 | 0 |
2 | 0.138 | 0.238 | 0.062 | 0.062 |
3 | c1 | c2 | c3 | c3 |
4 | c1 | c2 | c3 | c3 |
5 | 0.116 | 0.216 | 0.084 | |
6 | 0.096 | 0.266 | 0.104 |
��һ������ƽ��״̬��ʱ���Ϊ__��
��5��6minʱ����ڣ�ƽ���ƶ�����Ϊ__(������ƶ����������ƶ���)�����ݱ��������жϣ�ƽ���ƶ���ԭ����__(����ĸ���)��
a.������H2O(g)���� b.��������Ũ�� c.ʹ�ô��� d.�����¶�
��t2��ʱ(t2��t1)������ͬ�����·���������Ӧ����ƽ��ʱ��COŨ��__c1(�������������=��)��
��3����֪��ӦFe(s)+CO2(g)![]() FeO(s)+CO(g)��ƽ�ⳣ�����¶ȱ仯�����ͼ��ʾ��
FeO(s)+CO(g)��ƽ�ⳣ�����¶ȱ仯�����ͼ��ʾ��

�ٸ÷�Ӧ��__(����ȡ����ȡ�)��Ӧ��
����T1ʱˮú���任��Ӧ��ƽ�ⳣ������0.5����T1ʱFeO(s)+H2(g)![]() Fe(s)+H2O(g)��ƽ�ⳣ��Ϊ__��
Fe(s)+H2O(g)��ƽ�ⳣ��Ϊ__��
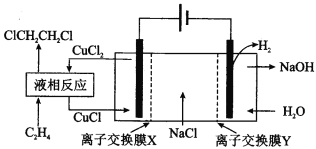
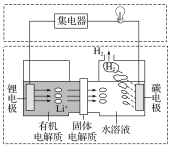
��4��ˮú������ȼ�ϵ�ص�ȼ�ϡ�һ������̼����ȼ�ϵ�صĹ���ԭ����ͼ��ʾ���缫A��H2����ĵ缫��ӦΪ__�������¯������CO��H2���ʵ���֮��Ϊ1�U2���缫A��������CO2�в��ֲ���ѭ�����ã���������Ϊ__��

����Ŀ����ͭ�ǹ㷺Ӧ�������������Ԫ�������úϽ�ij����С���ij�Ͼ���ͭԪ��(��25%BeO��71%CuS������ FeS�� SiO2)�л������ͭ���ֽ����Ĺ���������ͼ��
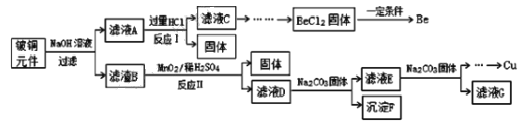
��֪��
��.�롢��Ԫ�ػ�ѧ�������ƣ�
��.�����²�����������ܶȻ����������
������ | Cu(OH)2 | Fe(OH)3 | Mn(OH)2 |
Ksp | 2.2��10-20 | 4.0��10-38 | 2.1��10-13 |
(1)��ҺA�ijɷֳ� NaOH��Na2BeO2�⣬����________(�ѧʽ)��д����ӦI��Na2BeO2����������ᷴӦ�Ļ�ѧ����ʽ��_________________��
(2)����ҺC ���ᴿ��BeCl2���������ʵ�鲽��˳��Ϊ_________(����ĸ)
a.��������İ�ˮ b.ͨ����� CO2 c.�������NaOH��Һ d.��������HCl e.ϴ�� f.����
(3)MnO2�ܽ����������е���Ԫ������Ϊ���ʣ�д����Ӧ����CuS������Ӧ�����ӷ���ʽ��__________������ŨHNO3�ܽ�������ȱ����________(��дһ��)
(4)��ҺD��c(Cu2+)= 2.2 molL-1��c(Fe3+) = 8.0��10 -3molL-1��c(Mn2+)= 0.01molL-1����μ��� Na2CO3��Һ����pH �ɽ���ת��������������η��룬���ȳ�������______ (�����ӷ���)��Ϊʹͭ���ӿ�ʼ������������Ӧ������Һ��pH____4��