ЬтФПФкШн
ЁОЬтФПЁПGaNЦОНшЦфГіЩЋЕФЙІТЪадФмЁЂЦЕТЪадФмвдМАЩЂШШадФмЃЌдкЙтЕчСьгђКЭИпЦЕЮЂВЈЦїМўгІгУЕШЗНУцгаЙуРЋЕФЧАОАЁЃ
(1) JohnsonЕШШЫЪзДЮдк1100ЁцЯТгУяигыАБЦјжЦЕУЕЊЛЏяиЃЌИУПЩФцЗДгІУПЩњГЩ1 mol H2ЗХГі10.3 kJШШСПЁЃИУЗДгІЕФШШЛЏбЇЗНГЬЪНЪЧ_____ЁЃ(МКжЊН№ЪєяиЕФШлЕуЪЧ29.8ЁцЃЌЗаЕуЪЧ2403ЁцЃЛЕЊЛЏяиЕФШлЕуЮЊ1700Ёц)
(2)дкКуШнУмБеШнЦїжаЃЌМгШывЛЖЈСПЕФвКЬЌяигыАБЦјЗЂЩњЩЯЪіЗДгІЃЌВтЕУЗДгІЦНКтЬхЯЕжаNH3ЕФЬхЛ§ЗжЪ§гыбЙЧП(p)ЁЂЮТЖШ(T)ЕФЙиЯЕШчЭМЫљЪО(вбжЊЭМжаT1КЭT2ЕФЮТЖШОљаЁгк1700Ёц)ЁЃ
ЂйЯТСаЫЕЗЈе§ШЗЕФЪЧ________(ЬюБъКХ)ЁЃ
a.ЯрЭЌЬѕМўЯТЃЌGa(OH)3 ЕФМюадБШAl(OH)3ЧП
b.ЕБc(NH3)=c(H2)ЪБЃЌвЛЖЈДяЕНСЫЛЏбЇЦНКтзДЬЌ
c. AЕуКЭCЕуЛЏбЇЦНКтГЃЪ§ЕФЙиЯЕЪЧЃКKA< KC
d.ЮТЖШвЛЖЈЪБЃЌДяЦНКтКѓдйГфШыКЄЦј(КЄЦјВЛВЮгыЗДгІ)ЃЌNH3ЕФзЊЛЏТЪдіДѓ.
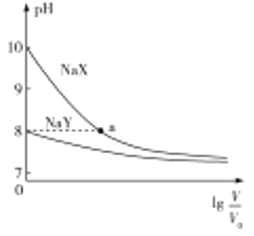
ЂкЦјЯрЦНКтжагУзщЗжЕФЦНКтЗжбЙДњЬцЮяжЪЕФСПХЈЖШвВПЩвдБэЪОЦНКтГЃЪ§(МЧзїKp)ЃЌвбжЊдкT1ЁцЪБЬхЯЕЕФбЙЧПГѕЪМбЙЧПЮЊa PaЃЌдђBЕуЕФKp=____(гУКЌaБэЪОЧвБЃСє2ЮЛгааЇЪ§зж)ЁЃ
(3)ЕчНтОЋСЖЗЈЬсДПяиЪЧЙЄвЕЩЯГЃгУЕФЗНЗЈЁЃОпЬхдРэШчЭМЫљЪОЃК
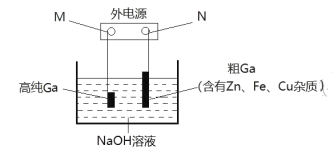
вбжЊЃКН№ЪєЕФЛюЖЏадZn>Ga>Fe>CuЃЛяиЛЏбЇаджЪгыТСЯрЫЦЁЃ
ЂйMЮЊЕчдДЕФ_______МЋЃЌЕчНтОЋСЖяиЪБВњЩњбєМЋФрЕФжївЊГЩЗжЪЧ________ЁЃ
ЂкЕчНтЙ§ГЬжабєМЋВњЩњЕФРызгЧЈвЦЕНДявѕМЋВЂдквѕМЋЮіГіИпДПяиЁЃЧыаДГіЕчНтЙ§ГЬЕФвѕМЋЕФЕчМЋЗДгІ__________ЁЃ
ЂлЕчНтЙ§ГЬжаашПижЦКЯЪЪЕФЕчбЙЃЌШєЕчбЙЬЋИпЪБвѕМЋЛсВњЩњH2ЕМжТЕчНтаЇТЪЯТНЕЁЃШєЭтЕчТЗЭЈЙ§0.2moleЪБЃЌвѕМЋЕУЕН3.5gЕФяиЁЃдђИУЕчНтзАжУЕФЕчНтаЇТЪІЧ=_________(ІЧ=ЩњГЩФПБъВњЮяЯћКФЕФЕчзгЪ§+зЊвЦЕФЕчзгзмЪ§)ЁЃ
ЁОД№АИЁП2Ga(l)+2NH3(g)=2GaN(s)+3H2(g) H= -30.9kJ/mol ac 1.7aPa ИКМЋ FeЁЂCu GaO2-+3e-+2H2O=Ga+4OH- 75%
ЁОНтЮіЁП
ЃЈ1ЃЉяигыАБЦјдк1100ЁцЯТЗДгІЩњГЩЕЊЛЏяиКЭЧтЦјЃЌаДГіЗНГЬЪНЃЌИљОнЩњГЩ1 mol H2ЗХГі10.3 kJШШСПЃЌМЦЫуТњзуЗНГЬЪНжаЧтЦјЕФЮяжЪЕФСПЕФШШСПЃЌМДПЩе§ШЗЪщаДИУШШЛЏбЇЗНГЬЪНЃЛ
ЃЈ2ЃЉЂйИљОнЭЌжїзхдЊЫиаджЪЕФЕнБфЙцТЩЁЂДяЕНЦНКтзДЬЌЕФБъжОЁЂЦНКтГЃЪ§гыЮТЖШЕФЙиЯЕЁЂЦНКтвЦЖЏЕФЬѕМўзїГіХаЖЯЃЛ
ЂкНсКЯЭМЯёаХЯЂЃЌ2Ga(l)+2NH3(g)=2GaN(s)+3H2(g)ЃЌНсКЯЦНКтЪБАБЦјЕФЬхЛ§ЗжЪ§ЃЌМЦЫуГіЦНКтЪБЕФзмбЙЧПЃЌШЛКѓМЦЫуГіАБЦјЁЂЧтЦјЕФЗжбЙЃЌзюКѓМЦЫуЗДгІЦНКтГЃЪ§ЃЛ
ЃЈ3ЃЉЕчНтОЋСЖЗЈЬсДПяиЃЌНсКЯДжЭЕФОЋСЖЃЌПЩвдХаЖЯГіДжяизїбєМЋЃЌИпДПяизївѕМЋЃЌаДбєМЋВњЮяЕФЪБКђвЊзЂвтяиЪЇШЅЕчзгБфЮЊяиРызгЃЌяиРызггыШмвКжаЕФЧтбѕИљРызгНсКЯЩњГЩGaO2-ЃЌШЛКѓGaO2-дквѕМЋЕУЕчзгЩњГЩяиЁЃ
ЃЈ1ЃЉяигыАБЦјдк1100ЁцЯТЗДгІЩњГЩЕЊЛЏяиКЭЧтЦјЃЌЗДгІЕФЗНГЬЪНЮЊ2Ga(l)+2NH3(g)=2GaN(s)+3H2(g)ЃЌЩњГЩ1 mol H2ЗХГі10.3 kJШШСПЃЌЙЪЩњГЩ3 mol H2ЗХГі30.9 kJШШСПЃЌИУШШЛЏбЇЗНГЬЪНЮЊ2Ga(l)+2NH3(g)=2GaN(s)+3H2(g) H= -30.9kJ/mol ЃЛ
ЃЈ2ЃЉЂйaЃЎяигыТСЮЛгкЭЌвЛжїзхЃЌЭЌвЛжїзхДгЩЯЕНЯТЃЌЫцзХКЫЕчКЩЪ§ЕФдіМгЃЌН№Ъєадж№НЅдіЧПЃЌН№ЪєаддНЧПЃЌзюИпМлбѕЛЏЮяЖдгІЕФЫЎЛЏЮяЕФМюаддНЧПЃЌЙЪЯрЭЌЬѕМўЯТЃЌGa(OH)3 ЕФМюадБШAl(OH)3ЧПЃЌaе§ШЗЃЛ
bЃЎЕБЗДгІЮяХЈЖШЛђЩњГЩЮяХЈЖШЫцзХЪБМфЕФИФБфЖјВЛБфЃЌЗДгІДяЕНЦНКтЃЌЕБc(NH3)=c(H2)ЪБЃЌЗДгІВЛвЛЖЈДяЕНСЫЛЏбЇЦНКтзДЬЌЃЌbДэЮѓЃЛ
cЃЎ2Ga(l)+2NH3(g)=2GaN(s)+3H2(g) H= -30.9kJ/molЃЌИУЗДгІЮЊЗХШШЗДгІЃЌЮТЖШдНИпЃЌЦНКтЪБАБЦјЕФЬхЛ§ЗжЪ§дНДѓЃЌВЂЧвЮТЖШдНИпЃЌЛЏбЇЦНКтГЃЪ§дНаЁЃЌНсКЯЭМЯёПЩжЊЃЌT1T2ЃЌKA< KCЃЌcе§ШЗЃЛ
dЃЎЬхЛ§ВЛБфЃЌЮТЖШвЛЖЈЪБЃЌДяЦНКтКѓдйГфШыКЄЦј(КЄЦјВЛВЮгыЗДгІ)ЃЌЗДгІЮяЁЂЩњГЩЮяЕФХЈЖШВЛБфЃЌЦНКтВЛвЦЖЏЃЌЙЪNH3ЕФзЊЛЏТЪВЛБфЃЌdДэЮѓЃЛ
Ђк2Ga(l)+2NH3(g)=2GaN(s)+3H2(g) ЃЌСюЗДгІПЊЪМЧАЯђШнЦїжаГфШыxmolАБЦјЃЌДяЕНЦНКтЪБАБЦјЕФзЊЛЏТЪЮЊbЃЌЙЪЦНКтЪБАБЦјЕФЮяжЪЕФСПЮЊx(1-b)molЃЌЧтЦјЕФЮяжЪЕФСПЮЊ1.5bxmolЃЌBЕуЪБЃЌАБЦјЕФЬхЛ§ЗжЪ§ЮЊ0.4ЃЌМД
![]() =0.4ЃЌb=0.5ЃЌЦНКтЪБАБЦјЕФЮяжЪЕФСПЮЊ0.5xmolЃЌЧтЦјЕФЮяжЪЕФСПЮЊ0.75xmolЃЌКуЮТКуШнЕФШнЦїжаЃЌШнЦїжазмбЙЧПжЎБШЕШгкЮяжЪЕФСПжЎБШЃЌЦНКтКѓЕФбЙЧПЮЊp6ЃЌxЃК(0.5x+0.75x)=aЃКp6ЃЌp6=1.25aPaЃЌЦНКтЪБАБЦјЕФЗжбЙЮЊ0.4ЁС1.25aPa=0.5aPaЃЌЧтЦјЕФЗжбЙЮЊ0.6ЁС1.25aPa=0.75aPaЃЌKp=
=0.4ЃЌb=0.5ЃЌЦНКтЪБАБЦјЕФЮяжЪЕФСПЮЊ0.5xmolЃЌЧтЦјЕФЮяжЪЕФСПЮЊ0.75xmolЃЌКуЮТКуШнЕФШнЦїжаЃЌШнЦїжазмбЙЧПжЎБШЕШгкЮяжЪЕФСПжЎБШЃЌЦНКтКѓЕФбЙЧПЮЊp6ЃЌxЃК(0.5x+0.75x)=aЃКp6ЃЌp6=1.25aPaЃЌЦНКтЪБАБЦјЕФЗжбЙЮЊ0.4ЁС1.25aPa=0.5aPaЃЌЧтЦјЕФЗжбЙЮЊ0.6ЁС1.25aPa=0.75aPaЃЌKp=![]() =1.7aPa ЃЛ
=1.7aPa ЃЛ
ЃЈ3ЃЉЂйЕчНтОЋСЖЗЈЬсДПяиЃЌДжяизїбєМЋЃЌИпДПяизївѕМЋЃЌдђMЮЊИКМЋЃЌNЮЊе§МЋЃЛгЩН№ЪєЕФЛюЖЏадZn>Ga>Fe>CuПЩжЊЃЌFeЁЂCuУЛгаGaЛюЦУЃЌдкбєМЋВЛЗЂЩњЗДгІЃЌZnЁЂGaдкбєМЋЗЂЩњЗДгІЃЌЙЪЕчНтОЋСЖяиЪБВњЩњбєМЋФрЕФжївЊГЩЗжЪЧFeЁЂCuЃЛ
ЂкGaгыAlдкжмЦкБэжаЮЛгкЭЌвЛжїзхЃЌЙЪGaдкбєМЋЕФЕчМЋЗДгІЪНЮЊGa-3e-+4OH-=GaO2-+2H2OЃЌвѕМЋЕФЕчМЋЗДгІЪНЮЊGaO2-+3e-+2H2O=Ga+4OH-ЃЛ
ЂлвѕМЋЕУЕН3.5gЕФяиЃЌn(Ga)=![]() =0.05molЃЌвѕМЋЕФЕчМЋЗДгІЪНЮЊGaO2-+3e-+2H2O=Ga+4OH-ЃЌгЩвѕМЋЕчМЋЗДгІЪНПЩжЊЃЌвѕМЋЕУЕН3.5gЕФяиЃЌЕУЕНЕФЕчзгЕФЮяжЪЕФСПЮЊ3ЁС0.05mol=0.15molЃЌЫљвдИУЕчНтзАжУЕФЕчНтаЇТЪІЧ=
=0.05molЃЌвѕМЋЕФЕчМЋЗДгІЪНЮЊGaO2-+3e-+2H2O=Ga+4OH-ЃЌгЩвѕМЋЕчМЋЗДгІЪНПЩжЊЃЌвѕМЋЕУЕН3.5gЕФяиЃЌЕУЕНЕФЕчзгЕФЮяжЪЕФСПЮЊ3ЁС0.05mol=0.15molЃЌЫљвдИУЕчНтзАжУЕФЕчНтаЇТЪІЧ=![]() =75% ЁЃ
=75% ЁЃ

ЁОЬтФПЁПАБЛљМзЫсяЇ(H2NCOONH4)ЪЧвЛжжвзЗжНтЁЂвзЫЎНтЕФАзЩЋЙЬЬхЃЌФГбаОПаЁзщвдЧтбѕЛЏФЦЙЬЬхЁЂХЈАБЫЎЁЂИЩБљЕШЮЊдСЯжЦБИАБЛљМзЫсяЇЕФЪЕбщзАжУШчЭМЫљЪОЃЌЦфжївЊЗДгІЕФдРэЮЊ2NH3(g)+CO2(g)NH2COONH4(s)ЁЁІЄHЃМ0ЁЃ
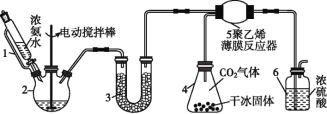
(1)вЧЦї3жаЪЂзАЕФЙЬЬхЪЧ____ЃЌЦфзїгУЪЧ________________ЁЃ
(2)вЧЦї6ЕФвЛИізїгУЪЧПижЦдСЯЦјАДЛЏбЇМЦСПЪ§ГфЗжЗДгІЃЌШєЗДгІГѕЦкЙлВьЕНзАжУФкХЈСђЫсжаВњЩњЦјХнЃЌдђгІИУ___(ЬюЁАМгПьЁБЁАМѕТ§ЁБЛђЁАВЛИФБфЁБ )ВњЩњАБЕФЫйТЪЁЃ
(3)СэвЛжжжЦБИАБЛљМзЫсяЇЕФЗДгІзАжУ(вКЬЌЪЏРЏКЭCCl4ОљГфЕБЖшадНщжЪ)ШчЭМЫљЪОЁЃ
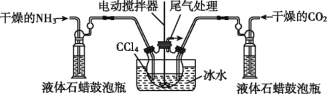
ЂйвКЬЌЪЏРЏЙФХнЦПЕФзїгУЪЧ________________________ЁЃ
ЂкЕБCCl4вКЬхжаВњЩњНЯЖрОЇЬхаќИЁЮяЪБЃЌСЂМДЭЃжЙЗДгІЃЌЙ§ТЫЗжРыЕУЕНДжВњЦЗЃЌЮЊСЫНЋЫљЕУДжВњЦЗИЩдяЃЌПЩВЩШЁЕФЗНЗЈЪЧ___(ЬюзжФИ)ЁЃ
AЃЎеєСѓЁЁЁЁЁЁЁЁЁЁЁЁ BЃЎецПеЮЂШШКцИЩЁЁЁЁ CЃЎИпбЙМгШШКцИЩ
(4)жЦЕУЕФАБЛљМзЫсяЇжаПЩФмКЌгаЬМЫсЧтяЇЁЂЬМЫсяЇжаЕФвЛжжЛђСНжждгжЪ(ВЛПМТЧАБЛљМзЫсяЇгыЫЎЕФЗДгІ)ЁЃ
ЂйЩшМЦЗНАИНјааГЩЗжЬНОПЃЌЧыЬюаДБэжаПеИёЁЃ
ЯобЁЪдМСЃКеєСѓЫЎЁЂЯЁЯѕЫсЁЂBaCl2ШмвКЁЂГЮЧхЪЏЛвЫЎЁЂAgNO3ШмвКЁЂЯЁбЮЫсЁЃ
ЪЕбщВНжш | дЄЦкЯжЯѓКЭНсТл |
ВНжш1ЃКШЁЩйСПЙЬЬхбљЦЗгкЪдЙмжаЃЌМгШыеєСѓЫЎжСЙЬЬхШмНт | ЕУЕНЮоЩЋШмвК |
ВНжш2ЃКЯђЪдЙмжаМгШыЙ§СПЕФBaCl2ШмвКЃЌОВжУ | ШєШмвКВЛБфЛызЧЃЌдђжЄУїЙЬЬхжаВЛКЌЬМЫсяЇ |
ВНжш3ЃКЯђЪдЙмжаМЬајМгШы____________ | _____________ЃЌдђжЄУїЙЬЬхжаКЌгаЬМЫсЧтяЇ |
ЂкИљОнЂйЕФНсТлЃЌШЁ15.8 gАБЛљМзЫсяЇбљЦЗЃЌгУзуСПЧтбѕЛЏБЕШмвКГфЗжДІРэКѓЃЌЙ§ТЫЯДЕгЁЂИЩдяЃЌВтЕУГСЕэЕФжЪСПЮЊ1.97 gЁЃдђбљЦЗжаАБЛљМзЫсяЇЕФжЪСПЗжЪ§ЮЊ____ЁЃ
ЁОЬтФПЁПЯТСаЪЧФГЭЌбЇЖдЯргІЗДгІЕФРызгЗНГЬЪНЫљзїЕФЦРМлЃЌЦфжаЖдгІЕФЦРМлКЯРэЕФЪЧЃЈ ЃЉ
БрКХ | ЛЏбЇЗДгІ | РызгЗНГЬЪН | ЦРМл |
A | АбMgSO4ШмвКЕЮШыBa(OH)2ШмвК | Mg2++2OH-=Mg(OH)2Ё§ | е§ШЗ |
B | бѕЛЏЭгыЯЁбЮЫсЗДгІ | CuO+2H+=Cu2++H2O | ДэЮѓЃЌВЛЗДгІ |
C | ЯђFeCl2ШмвКжаЭЈШыТШЦј | Fe2++Cl2=Fe3++2Cl- | ДэЮѓЃЌЕчКЩВЛЪиКу |
D | ЯђЗаЫЎЕЮШыБЅКЭТШЛЏ ЬњШмвК | Fe3++3H2O | е§ШЗ |
A.AB.BC.CD.D