��Ŀ����
8��ij��ѧʵ��С������50mLNaOH��Һ����CO2���壨�ô���ʯ��ϡ���ᷴӦ����ȡCO2�������Ʊ�������Na2CO3��Һ��Ϊ�˷�ֹͨ���CO2�����������NaHCO3���������������ʵ����̣�a��ȡ25mL NaOH��Һ��������ͨ�������CO2���壬��CO2���岻���ܽ⣻
b��С����д���Һ1��2min��
c���ڵõ�����Һ�м�����һ�루25mL��NaOH��Һ��ʹ���ֻ�Ϸ�Ӧ��
��1���˷������Ƶýϴ�����Na2CO3��д��a��c�����Ļ�ѧ��Ӧ����ʽ��NaOH+CO2�TNaHCO3��NaHCO3+NaOH�TNa2CO3+H2O��
��2�������Һ��Ŀ���dz�ֽ���Һ�еĶ�����̼�ϳ����˷�����һ����ʵ��װ����ͼ��ʾ��

��3�����뷴Ӧ��ǰ����μ������װ�õ��������õ��ɼм�סA��B���Ӵ����ȼ��A�����ԣ�������Ƥ������©��ע��һ������ˮ��ʹ©���е�ˮ�������ƿ�ڵ�ˮ�棬ֹͣ��ˮ��©��������ƿ�е�Һ���ֲ��䣬˵��װ�ò�©����Ȼ����B�������ԣ����ձ���ע������ˮ��ʹ���ܿ�����ˮ�У�˫����ס���ƿƬ��������ð�����ɿ��ֺ�������ˮ���뵼���γ�ˮ����˵��װ�ò�©����Ҳ��һ�μ��A��B�������ԣ����Ӻ��ձ�����齺����ֹˮ�м�ס��Ȼ���©��ע��һ������ˮ��ʹ©���е�ˮ�������ƿ�ڵ�ˮ�棬��һ�ᣬ�۲�©��������ƿ�е�Һ�������ֲ��䣬˵��װ�ò�©������
��4��װ��B��ʢ�ŵ��Լ��DZ���NaHCO3��Һ������������HCl���壮
��5����ʵ�����Ʒ��У�װ��A������Ϊ���Тڢܢ�����ķ���װ�ã�����ţ���
��CH2=CH2 ��H2S ��CH4 ��CH��CH ��H2
��6��ʵ������ȡ�������壺��NH3����Cl2����HCl����H2S����CH4����CO����CO2����O2ʱ�����ڱ������β������������ͼ��ʾװ�ý��д����ģ���������������װ��ͼ���·��ո��ڣ�
| β �� �� �� װ �� |  | 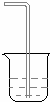 |
| �������� | �٢� | �ڢ� |
���� ��1������NaOH��CO2��Ӧ��CO2��������NaHCO3����д��ѧ����ʽ��������ʽ������Ӧ�����κ�ˮ��
��2�������Һ��Ŀ���ǸϾ���Һ�еĶ�����̼��������ź���ʵ������
��3������װ�ò�©��ʱ���ı������������Ըı�ѹǿ��
��4������CO2�к���HCl��ѡ������Լ���
��5������װ�õ��ص㣺����+Һ������壻
��6����������װ�õ��ص㣺ǰ���Ƿ�������˵�����弫������ˮ������Һ��Ӧ������û�з�������˵�����岻����ˮ������Һ��Ӧ��
��7���ȸ���NaOH��CO2��Ӧ����NaHCO3�����NaHCO3�����ʵ������ٸ��ݷ�Ӧ��NaHCO3+NaOH�TNa2CO3+H2O�����Na2CO3�����ʵ������������Na2CO3�����ʵ���Ũ�ȣ�
��� �⣺��1���������CO2��NaOH��Һ��Ӧ����NaHCO3���䷴ӦΪNaOH+CO2�TNaHCO3������ʽ����Ӧ�����κ�ˮ���䷴ӦΪNaHCO3+NaOH�TNa2CO3+H2O��
�ʴ�Ϊ��NaOH+CO2�TNaHCO3��NaHCO3+NaOH�TNa2CO3+H2O��
��2�������Һ��Ŀ���ǸϾ���Һ�еĶ�����̼��������ź���ʵ������
�ʴ�Ϊ����ֽ���Һ�еĶ�����̼�ϳ���
��3����װ�ò�©��ʱ��ͨ���ı������������Ըı�ѹǿ���õ��ɼм�סA��B���Ӵ����ȼ��A�����ԣ�������Ƥ������©��ע��һ������ˮ��ʹ©���е�ˮ�������ƿ�ڵ�ˮ�棬ֹͣ��ˮ��©��������ƿ�е�Һ���ֲ��䣬˵��װ�ò�©����Ȼ����B�������ԣ����ձ���ע������ˮ��ʹ���ܿ�����ˮ�У�˫����ס���ƿƬ��������ð�����ɿ��ֺ�������ˮ���뵼���γ�ˮ����˵��װ�ò�©����
�ʴ�Ϊ���õ��ɼм�סA��B���Ӵ����ȼ��A�����ԣ�������Ƥ������©��ע��һ������ˮ��ʹ©���е�ˮ�������ƿ�ڵ�ˮ�棬ֹͣ��ˮ��©��������ƿ�е�Һ���ֲ��䣬˵��װ�ò�©����Ȼ����B�������ԣ����ձ���ע������ˮ��ʹ���ܿ�����ˮ�У�˫����ס���ƿƬ��������ð�����ɿ��ֺ�������ˮ���뵼���γ�ˮ����˵��װ�ò�©����Ҳ��һ�μ��A��B�������ԣ����Ӻ��ձ�����齺����ֹˮ�м�ס��Ȼ���©��ע��һ������ˮ��ʹ©���е�ˮ�������ƿ�ڵ�ˮ�棬��һ�ᣬ�۲�©��������ƿ�е�Һ�������ֲ��䣬˵��װ�ò�©������
��4����CO2�к���HCl��Ҫ�õ�������CO2�������ȥHCl��ͬʱ�����Լ�����CO2��Ӧ�������ñ���NaHCO3��Һ�������ñ���Na2CO3��Һ��
�ʴ𰸣�����NaHCO3��Һ������HCl���壻
��5�����Ʊ�CO2��H2S��CH��CH��H2װ�õ��ص㣺����+Һ������壬���Ʊ�CH2�TCH2 װ�õ��ص㣺Һ��+Һ������壬�Ʊ�CH4װ�õ��ص㣺����+��������壬�ʴ�Ϊ���ڢܢݣ�
��6�����ж�����������β����������CH4��CO2��O2����Ҫ������CO��Ȼ��Ҫ��������������Һ�������գ�����ȼ�շ�����������ߵ�װ����������Ҫ��������˵�����弫������ˮ���磺NH3��HCl �ұߵ�װ�ú�����������û�з�������˵�����岻������ˮ���磺Cl2��H2S���ʴ�Ϊ���٢ۣ��ڢܣ�
��7��50mLNaOH��Һ��n��NaOH��=$\frac{50ml��1.44g/ml��40%}{40g/mol}$=0.72mol��
NaOH+CO2�TNaHCO3
0.36mol 0.36mol
NaHCO3 +NaOH�TNa2CO3 +H2O
0.36mol 0.36mol 0.36mol
��Na2CO3�����ʵ���Ũ��Ϊ��C=$\frac{n}{V}$=$\frac{0.36mol}{0.05L}$=7.2 mol/L��
�ʴ𰸣�7.2 mol/L��
���� ������һ��ʵ���ۺ��⣬��Ҫ�����ʵ��Ʊ�������ʵ����ƣ�ʵ������жϺͷ�Ӧ�Ķ������㣬������ѧ����ʵ��֪ʶ��ͬʱ������ѧ���Ļ�ѧ������������һ�����Ѷȣ����ջ����ǹؼ���

| A�� | SO42- | B�� | CO32- | C�� | NO3- | D�� | Br- |
| A�� | �ɱ�ʾ����ͭ�κ�ǿ��ķ�Ӧ | |
| B�� | �ɱ�ʾijһ������ķ�Ӧ��Ҳ���Ա�ʾһ�෴Ӧ | |
| C�� | ���ӷ���ʽ�е�OH-�ɴ��������ǿ�� | |
| D�� | �÷�Ӧ�ɿ���Cu��OH��2��ɫ���� |
| A�� | B2Ϊ0.1mol/L | B�� | DΪ0.3mol/L | ||
| C�� | A2Ϊ0.3mol/L | D�� | B2Ϊ0.2mol/LͬʱDΪ0.1mol/L |
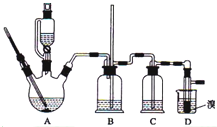 ʵ�����Ʊ�1��2-��������ķ�Ӧԭ�����£�
ʵ�����Ʊ�1��2-��������ķ�Ӧԭ�����£�CH3CH2OH $��_{170��}^{Ũ����}$CH2=CH2��+H2O
CH2=CH2+Br2��BrCH2CH2Br
���ܴ��ڵ���Ҫ����Ӧ�У��Ҵ���Ũ����Ĵ�������l40����ˮ�������ѣ�������������������Ҵ��Ʊ�1��2-���������װ����ͼ��ʾ���й������б����£�
| �Ҵ� | 1��2-�������� | ���� | |
| ״̬���ܽ��� | ��ɫҺ�塢������ˮ | ��ɫҺ�� | ��ɫҺ�塢������ˮ |
| �ܶ�/g•cm-3 | 0.79 | 2.2 | 0.71 |
| �е�/�� | 78.5 | 132 | 34.6 |
| �۵�/�� | -l30 | 9 | -1l6 |
��1���ڴ��Ʊ�ʵ���У�Ҫ������Ѹ�ٵذѷ�Ӧ�¶����170�����ң�������ҪĿ����d��
a��������Ӧ b���ӿ췴Ӧ�ٶ� c����ֹ�Ҵ��ӷ� d�����ٸ�������������
��2���жϸ��Ʊ���Ӧ�Ѿ�������������������ɫ��ȫ��ȥ��
��3����1��2-��������ֲ�Ʒ���ڷ�Һ©���м�ˮ�����ã�����Ӧ�ڲ��£���ϡ������¡�����
��4�������������������������ѣ����õķ�����ȥ����
��5����Ӧ������Ӧ����ˮ��ȴװ��D������ҪĿ���ǣ����ֲ��ܹ�����ȴ�����ñ�ˮ������ԭ���DZ���������ӷ�����Ʒ1��2-����������۵㣨���̵㣩�ͣ�������ȴ�����̶��������ܣ�
 S2Cl2�ǹ�ҵ�ϳ��õ��������Ըı��������ȷ�ճ�������Ӳ�����ʣ�
S2Cl2�ǹ�ҵ�ϳ��õ��������Ըı��������ȷ�ճ�������Ӳ�����ʣ�| ���� | �е�/�� | �۵�/�� |
| S | 445 | 113 |
| CS2 | 47 | -109 |
| CCl4 | 77 | -23 |
| S2Cl2 | 137 | -77 |
ʵ�����Ʊ�S2Cl2�ķ�Ӧԭ�������֣�
��CS2+3C12$\frac{\underline{\;95��100��\;}}{\;}$ CC14+S2Cl2��
��2S+Cl2$\frac{\underline{\;111��140��\;}}{\;}$ S2Cl2��
��1����ʵ����ѡ��ͼװ�ã����ּг�װ������ȥ�����Ʊ�S2Cl2���䷴Ӧԭ���������еĢ٣���д��ţ���ͼ��β������װ�ò������ƣ��Ľ����װ��Ӧ�������ǣ���D��E֮������װ�������β�����ա������������ã����øĽ������ȷװ�ý���ʵ�飬��ش��������⣺
��2��Ϊ����߲�ƷS2Cl2�Ĵ��ȣ�ʵ������Ĺؼ��ǿ��ƺ��¶ȺͿ���Ũ����ĵ��ٲ�Ҫ̫�죮
��3����ʵ��IJ���˳��Ϊ���٢ۢڢݢܣ�����ű�ʾ����
�ٵ�ȼ�ƾ���A �ڵ�ȼ�ƾ���D ��ͨ����ˮ ��Ϩ��ƾ���A ��Ϩ��ƾ���D
��4��ijͬѧȡ�������С�ļ�������ˮ�У��۲쵽�а�������ɫ�����Ҳ�������ɫ������ʹƷ����Һ��ɫ����ͬѧ�ݴ��ж�����ʵ��ȷ��S2Cl2���ɣ�д��S2Cl2��ˮ��Ӧ�Ļ�ѧ����ʽ��2S2Cl2+2H2O�T3S��+SO2��+4HCl����
��5��S2Cl2��ÿ��ԭ�ӵ�����㶼����8���ӽṹ�����õ���ʽ��ʾS2Cl2���γɹ��̣�
 ��
�� 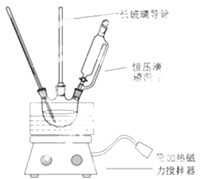 ����������Ҫ�ľ�ϸ����ԭ�ϣ���ҽҩ��Ⱦ�ϵ��м��壬�������л��ܼ����Ʊ�����������װ��ͼ��Ӧװ�ã��������£�
����������Ҫ�ľ�ϸ����ԭ�ϣ���ҽҩ��Ⱦ�ϵ��м��壬�������л��ܼ����Ʊ�����������װ��ͼ��Ӧװ�ã��������£� ��
��