��Ŀ����
����Ŀ����1��������һ��ǿ�ᣬ�����Ũ�ȳ���40%�ͻ�Ѹ�ٷֽ⣬�����ж����壬��Ӧ�Ļ�ѧ����ʽΪ8HClO3=3O2����2Cl2����4HClO4��2H2O�����û�������ƽ����Է�������Ϊ________��
��2��ʵ���ҿ������̿���Ҫ�ɷ�ΪMnO2����ȡKMnO4���������£�������ʹ���̿������KOH(s)��KClO3 (s)��Ӧ������K2MnO4(�����)��KCl����ˮ�ܽ⣬��ȥ�������ữ��Һ��K2MnO4ת��ΪMnO2��KMnO4������ȥ����MnO2��Ũ���ᾧ�õ�KMnO4���塣K2MnO4ת��ΪKMnO4�ķ�Ӧ���������ͻ�ԭ�������ʵ���֮��Ϊ________��
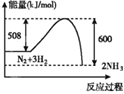
��3����֪�ⶨ�̵�һ�ַ����ǣ�������ת��Ϊ����������ӣ���Ӧ��ϵ����H����Mn2����H2O��![]() ��
��![]() ��
��![]() ��
��
���÷�Ӧ�����ӷ���ʽΪ________________________����������ת��Ϊ����������ӵķ�Ӧ�У�����ѷ�Ӧ�����Һϡ�͵�1 L�������Һ��pH��2�����ڷ�Ӧ��ת�Ƶ��ӵ����ʵ���Ϊ________ mol�������д��С����������λ��Ч���֣�
���ڲⶨ������Ũ�ȵ�ʵ���У���Ҫ����250 mL 0.10mol/L KIO4�ı���Һ��Ӧ��������ƽ��ȡ ______g KIO4���塣
�������й�������Һ�IJ������ж���ȷ������_________��
A��ʹ������ƿǰ����������ƿ�Ƿ�����Լ�ƿ�����Ƿ�©ˮ
B������ƿ������ˮϴ����û�к�ɾ�����������Һ
C������ҡ�Ⱥ�Һ���½��ټ�ˮ���̶��ߣ���Ӱ��������ҺŨ��
D������ʱ���ӿ̶��ᵼ��������ҺŨ��ƫ��
E��������ƿ�е�Һ�����̶���2~3cmʱ���ý�ͷ�ιܼ�ˮ����
���𰸡�47.6 1��2 ![]() 0.0167 5.8 A��B
0.0167 5.8 A��B
��������
(1)��������ƽ����Է�����������ֵ��=ƽ��Ħ������=![]() ��
��
(2) K2MnO4ת��ΪKMnO4�ķ�Ӧ��K2MnO4��KMnO4+ MnO2��MnԪ�صĻ��ϼۼ������ֽ��ͣ�
(3)��������ʧ����ת��Ϊ����������ӣ�����������Ϊ��ԭ������õ��ӻ��ϼ۽��͵�������������������Ԫ�صĻ��ϼ�ȷ���������ͻ�ԭ����ٽ�����ӷ���ʽ����д������д����Ӧ�����Һϡ�͵�1L�������Һ��pH=2����n(H+)=1L0.01mol/L=0.01mol�����ݷ���ʽ���㣻
������һ�����ʵ���Ũ����Һʱ������������m=nM=cVM��
�۸�������һ�����ʵ���Ũ����Һ��ע�����������
�������Ϸ������
(1) HClO3�ֽ����ɵ�����ΪO2��Cl2�����ʵ���֮��Ϊ3��2����������ƽ��Ħ������Ϊ![]() =
=![]() =47.6g/mol���������û�������ƽ����Է�������Ϊ47.6��
=47.6g/mol���������û�������ƽ����Է�������Ϊ47.6��
����47.6��
(2) K2MnO4ת��ΪMnO2��KMnO4�ķ�Ӧ�У�K2MnO4��KMnO4+ MnO2��MnԪ�صĻ��ϼۼ������ֽ��ͣ�����K2MnO4�������������ǻ�ԭ����MnԪ�صĻ��ϼ���+6�۽���+4�۷�����ԭ��Ӧ��Ϊ��������MnԪ�صĻ��ϼ���+6�����ߵ�+7�۷���������Ӧ��Ϊ��ԭ������ʧ������֮��Ϊ2��1�����ݵ�ʧ�����غ㣬�������뻹ԭ�������ʵ���֮��Ϊ1��2��
����1��2��
(3)��������ת��Ϊ����������ӣ�����������Ϊ��ԭ�����������õ��ӻ��ϼ۽��ͣ�IO3-��IO4-�е�Ԫ�صĻ��ϼ۷ֱ���+5�ۺ�+7�ۣ�����IO4-������������ԭ������IO3-��ͬʱˮ�μӷ�Ӧ���������ӣ����Ը÷�Ӧ�����ӷ���ʽΪ��2Mn2++5IO4-+3H2O=2MnO4-+5IO3-+6H+��
�ɷ�Ӧ2Mn2++5IO4-+3H2O=2MnO4-+5IO3-+6H+��֪������Ӧ��ת��10mol����ʱ������6mol�����ӣ�����ѷ�Ӧ�����Һϡ�͵�1 L�������Һ��pH��2������n(H+)=1L0.01mol/L=0.01mol������ת�Ƶ���Ϊ![]() =0.0167mol��
=0.0167mol��
����2Mn2++5IO4-+3H2O=2MnO4-+5IO3-+6H+��0.0167 ��
������һ�����ʵ���Ũ����Һ��ʵ��ĵ�һ���Ǽ����������ʵ���������Ҫ����250 mL 0.10mol/L KIO4�ı���Һ����ҪKIO4���������m= nM=cVM=0.1 mol/L25010-3L230g/mol=5.8g��
����5.8��
�� A��ʹ������ƿǰ����������ƿ�Ƿ�����Լ�ƿ�����Ƿ�©ˮ��A��ȷ��
B������ƿ����������ˮ��������Ӱ����Һ�����ƣ�B��ȷ��
C������ҡ�Ⱥ�Һ���½�����Ϊ�в�����Һճ������ƿ�ڱ��ϣ��ټ�ˮ���̶�����Һ���������ʵ���Ũ�ȱ�С��C����
D������ʱ���ӿ̶��ᵼ��Һ�泬�����ߣ���Һ���ƫ��������ҺŨ��ƫ�ͣ�D����
E��������ƿ�е�Һ�����̶���1~2cmʱ���ý�ͷ�ιܼ�ˮ���ݣ�E����
����A��B��