��Ŀ����
����Ŀ��(1)��̬��ԭ�ӵļ۲�����Ų�ʽ���ܱ�ʾΪ2s22px22py2����Ϊ��Υ����___________ԭ�������
(2)��̬Cuԭ�Ӻ�������Ų�ʽΪ_______��������µ��ȶ���CuO _____ Cu2O(����������������)��
(3)������CO(NH2)2��C��N���ӻ���ʽ�ֱ�Ϊ________��OF2�Ŀռ乹����___________��
(4)��Fe(NO3)3��Һ�м���Na2SO3����Һ�����ػ�ɫ��Ϊdz��ɫ����һ���ֱ�Ϊ�ػ�ɫ���ֱ�Ϊ�ػ�ɫ��ԭ����_____(�����ӷ���ʽ��ʾ)��
(5)������(NH3BH3)����Ϊ�����DZ�������ʹ������֮һ�������д�����λ����д��������Ľṹʽ_____����д��һ���백���黥Ϊ�ȵ�����ķ���______(�ѧʽ)��
���𰸡����� [Ar]3d104s1��1s22s22p63s23p63d104s1 �� sp2��sp3 V�� 3Fe2++NO3-+4H+=3Fe3++NO��+2H2O  C2H6
C2H6
��������
(1)���ع���ԭ�Ӻ��������������ͬ�ĸ���������Ų�ʱ�ٵ��Ӿ����ܷ�ռ��ͬ��ԭ�ӹ����������״̬��ͬ��
(2)���ݹ���ԭ����д��̬Cuԭ�Ӻ�������Ų�ʽ��������������CuԪ�ص�ԭ�Ӻ�������Ų����ȶ�������
(3)����������C��Nԭ�ӳɼ���������VSEPRģ���жϣ����ݼ۲���ӶԻ������ۺ��ӻ���������ж�OF2�Ŀռ乹�ͣ�
(4)��������������H+��NO3-���������ԣ���������Fe2+�����жϣ�
(5)Bԭ���ṩ�չ����NH3��Nԭ���ṩ1�Թµ��Ӷԣ�ԭ��������ȡ��۵�������Ҳ��ȵ�����Ϊ�ȵ����塣
(1)��̬��ԭ�Ӽ۲�����Ų�ʽд��2s22px22py2����ԭ�Ӻ�������Ų�û����pz���������Υ�����ع���
(2)Cu��29��Ԫ�أ����ݹ���ԭ����֪��̬Cuԭ�Ӻ�������Ų�ʽ1s22s22p63s23p63d104s1���дΪ[Ar]3d104s1��CuO��Cu�ļ۲�����Ų�ʽ��3d94s0��3d��������ȶ�״̬����Cu2O��Cu�ļ۲�����Ų�ʽ��3d10��d�����ȫ�������ȶ�״̬�������ڸ���ʱCuO�ֽ��Ϊ�ȶ���Cu2O��������ʵ��ȶ���CuO��Cu2O��
(3)���ؽṹʽ��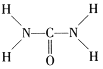 ����ͼ�п�֪��Cԭ��������4������ȫ������ɼ�����һ��C=O˫����VSEPRģ��Ϊƽ�������Σ�����Cԭ�Ӳ�ȡsp2�ӻ���Nԭ��������5����������3������ɼ�������һ�Թµ��Ӷԣ�VSEPRģ��Ϊ�����壬Nԭ�Ӳ�ȡsp3�ӻ���OF2��Oԭ��������6����������2���γɻ�ѧ����VSEPRģ��Ϊ�������Σ�Oԭ������2�Թµ��Ӷ�ռ��2��������µ��ӶԶԳɼ����ӶԵ��ų����ô��ڳɼ����ӶԵ��ų����ã�ʹOF2�Ŀռ乹����V�Σ�
����ͼ�п�֪��Cԭ��������4������ȫ������ɼ�����һ��C=O˫����VSEPRģ��Ϊƽ�������Σ�����Cԭ�Ӳ�ȡsp2�ӻ���Nԭ��������5����������3������ɼ�������һ�Թµ��Ӷԣ�VSEPRģ��Ϊ�����壬Nԭ�Ӳ�ȡsp3�ӻ���OF2��Oԭ��������6����������2���γɻ�ѧ����VSEPRģ��Ϊ�������Σ�Oԭ������2�Թµ��Ӷ�ռ��2��������µ��ӶԶԳɼ����ӶԵ��ų����ô��ڳɼ����ӶԵ��ų����ã�ʹOF2�Ŀռ乹����V�Σ�
(4)��Fe(NO3)3��Һ�м���Na2SO3��Һ��Fe3+��SO32-��H2O�ᷢ��������ԭ��Ӧ��2Fe3++SO32-+H2O=2Fe2++SO42-+2H+��ʹ��Һ�����ػ�ɫ��Ϊdz��ɫ��������Һ��ͬʱ����NO3-��������������H+��NO3-����������ã�����ǿ�������ԣ��ܹ���Fe2+����ΪFe3+���÷�Ӧ�����ӷ���ʽΪ��3Fe2++NO3-+4H+=3Fe3++NO��+2H2O����˹�һ����Һ����dz��ɫ��Ϊ�ػ�ɫ��
(5)Bԭ���������3�����ӣ��ܹ��ṩ�չ����NH3��Nԭ������һ�Թµ��Ӷԣ������ṩ1�Թµ��Ӷԣ�B��Nԭ��֮���γ���λ������˰�����(NH3BH3)�ĽṹʽΪ�� �����ڵȵ�������ԭ�Ӹ�����ͬ��ԭ�Ӻ���۵�������Ҳ��ͬ�����백���黥Ϊ�ȵ�����ķ��ӣ�������2��Cԭ�Ӵ���B��Nԭ�ӣ��백����ȵ�����һ�ַ���Ϊ��C2H6��
�����ڵȵ�������ԭ�Ӹ�����ͬ��ԭ�Ӻ���۵�������Ҳ��ͬ�����백���黥Ϊ�ȵ�����ķ��ӣ�������2��Cԭ�Ӵ���B��Nԭ�ӣ��백����ȵ�����һ�ַ���Ϊ��C2H6��

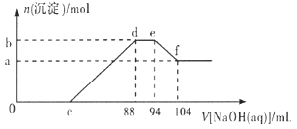
 ѧ�����νӽ̲��Ͼ���ѧ������ϵ�д�
ѧ�����νӽ̲��Ͼ���ѧ������ϵ�д�����Ŀ��ijѧ������֪���ʵ���Ũ�ȵ��������ⶨδ֪���ʵ���Ũ�ȵ�NaOH��Һʱ��ѡ�������ָʾ������ش��������⣺
��1���۲�ͼ����Һ����Ӧ����__�ζ����С�(����������������)

��2���ñ�������ζ������NaOH��Һʱ����������ʽ�ζ��ܵĻ���������ҡ����ƿ���۾�ע��__���ζ��յ������Ϊ__��
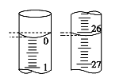
��3�����ζ���ʼ�ͽ���ʱ����ʽ�ζ����е�Һ����ͼ��ʾ������ʼ����Ϊ__mL������������Һ�����Ϊ___mL��
��4��ijѧ������3��ʵ��ֱ��¼�й����������ʾ��
�ζ����� | ����NaOH��Һ����� | 0.1000mol/L��������/mL | ||
�ζ�ǰ�̶� | �ζ���̶� | ��Һ���/mL | ||
��һ�� | 25.00 | 0.00 | 26.11 | 26.11 |
�ڶ��� | 25.00 | 1.56 | 30.30 | 28.74 |
������ | 25.00 | 0.22 | 26.31 | 26.09 |
���ݱ������ݼ����NaOH��Һ�����ʵ���Ũ��__��
��5�����в����п���ʹ����NaOH��Һ��Ũ����ֵƫ�͵���__(����ĸ���)��
A.��ʽ�ζ���δ�ñ�������ϴ��ֱ��ע�������
B.�ζ�ǰʢ��NaOH��Һ����ƿ������ˮϴ����û�и���
C.��ʽ�ζ����ڵζ�ǰ�����ݣ��ζ���������ʧ
D.��ȡ�������ʱ����ʼ���Ӷ������ζ�����ʱ���Ӷ���