��Ŀ����
����Ŀ���Ի���̿Ϊ���������Խ���ҵ�����е�SO2��������Ϊ���ᣬ�����Ƶø��������Σ������������[(NH4)2SO4��FeSO4��6H2O]�ȡ�
��1���о�������SO2�ڻ���̿�ϴ������ķ�Ӧ�������Ա�ʾΪ��*��ǵ�Ϊ����״̬��
���������������SO2(g)![]() SO2* ��H1=a kJ��mol��1
SO2* ��H1=a kJ��mol��1
��������������O2(g)![]() 2O* ��H2=b kJ��mol��1
2O* ��H2=b kJ��mol��1
�����������������SO2*+O*=SO3* ��H3=c kJ��mol��1
��������������Ѹ���SO3*![]() SO3(g) ��H4=d kJ��mol��1
SO3(g) ��H4=d kJ��mol��1
����������Ӧ������д��SO2�ڻ���̿�ϴ��������Ȼ�ѧ����ʽ____��
��2�������������[(NH4)2SO4��FeSO4��6H2O]Ϊԭ��ͨ���������̿����Ʊ�����A��

����֪25 ��ʱ��[Fe(C2O4)3]3��(aq)��SCN��(aq)![]() [Fe(SCN)]2��(aq)��3C2O42-(aq)��K��10��16��ij�о�С��ͬѧ�����龧��A�к�����������ȡ������������Թ��У�������ˮ����ܽ⣬���Թ��е��뼸��0.1 mol��L��1KSCN��Һ�����ж�����ʵ�鷽���Ƿ���в�˵�����ɣ�____��
[Fe(SCN)]2��(aq)��3C2O42-(aq)��K��10��16��ij�о�С��ͬѧ�����龧��A�к�����������ȡ������������Թ��У�������ˮ����ܽ⣬���Թ��е��뼸��0.1 mol��L��1KSCN��Һ�����ж�����ʵ�鷽���Ƿ���в�˵�����ɣ�____��
��ij�о�С��ͨ������ʵ�鲽��ⶨ����A�Ļ�ѧʽ��
����1��ȷ��ȡA��Ʒ4.910 0 g��������ˮ�����أ�����������Ϊ4.370 0 g��
����2��ȷ��ȡA��Ʒ4.910 0 g������ƿ�У�����������3.000 mol��L��1 H2SO4��Һ����������ˮ����0.500 0 mol��L��1 KMnO4��Һ�ζ�����MnO4��ǡ����ȫ����ԭΪMn2��ʱ������KMnO4��Һ�����Ϊ24.00 mL��(C2O42����MnO4����H+��CO2����Mn2����H2O)
����3��������1���ù�������ˮ����������0.280 0 g��ǡ����ȫ��Ӧ��
ͨ������ȷ������A�Ļ�ѧʽ��д��������̣���_____________
���𰸡�2SO2(g)+O2(g)![]() 2SO3(g) ��H=(2a+b+2c+2d)kJ��mol��1 �����У���Ϊ[Fe(C2O4)3]3��ת��Ϊ[Fe(SCN)]2����Ӧ��ƽ�ⳣ����С������������Ũ��̫С���۲첻�������������������� K3Fe(C2O4)3��3H2O
2SO3(g) ��H=(2a+b+2c+2d)kJ��mol��1 �����У���Ϊ[Fe(C2O4)3]3��ת��Ϊ[Fe(SCN)]2����Ӧ��ƽ�ⳣ����С������������Ũ��̫С���۲첻�������������������� K3Fe(C2O4)3��3H2O
��������
���ø�˹������дSO2�ڻ���̿�ϴ��������Ȼ�ѧ����ʽ��ƽ�ⳣ��С��![]() ��һ����Ϊ��Ӧ���ܽ��У����������غ���㾧��A�Ļ�ѧʽ��
��һ����Ϊ��Ӧ���ܽ��У����������غ���㾧��A�Ļ�ѧʽ��
��1����֪��
���������������SO2(g)![]() SO2* ��H1=a kJ��mol��1
SO2* ��H1=a kJ��mol��1
��������������O2(g)![]() 2O* ��H2=b kJ��mol��1
2O* ��H2=b kJ��mol��1
�����������������SO2*+O*=SO3* ��H3=c kJ��mol��1
��������������Ѹ���SO3*![]() SO3(g) ��H4=d kJ��mol��1
SO3(g) ��H4=d kJ��mol��1
���ݸ�˹���ɢ��2�����2������2��2SO2(g)+O2(g)![]() 2SO3(g) ��H=(2a+b+2c+2d)kJ��mol��1��
2SO3(g) ��H=(2a+b+2c+2d)kJ��mol��1��
��2����[Fe(C2O4)3]3��(aq)��SCN��(aq)![]() [Fe(SCN)]2��(aq)��3C2O42-(aq)��K��10��16�����ڷ�Ӧ��ƽ�ⳣ����С������������Ũ��̫С���۲첻�������������������飻
[Fe(SCN)]2��(aq)��3C2O42-(aq)��K��10��16�����ڷ�Ӧ��ƽ�ⳣ����С������������Ũ��̫С���۲첻�������������������飻
�� 4.910 0 g A��Ʒ��������ˮ�����أ�����������Ϊ4.370 0 g��˵���ᾧˮ��������4.910 0 g��4.370 0 g=0.540 0 g���ᾧˮ�����ʵ�����![]() ��������1���ù�������ˮ����������0.280 0 g��ǡ�÷�Ӧ����֪4.910 0 g����A�к���+3�۵������Ҿ�������Ԫ�ص����ʵ�����
��������1���ù�������ˮ����������0.280 0 g��ǡ�÷�Ӧ����֪4.910 0 g����A�к���+3�۵������Ҿ�������Ԫ�ص����ʵ�����![]() �����ݲ���2��5C2O42����2MnO4����16H+��10CO2����2Mn2����8H2O����Ӧ����0.500 0 mol��L��1 KMnO4��Һ24.00 mL����4.910 0 g
�����ݲ���2��5C2O42����2MnO4����16H+��10CO2����2Mn2����8H2O����Ӧ����0.500 0 mol��L��1 KMnO4��Һ24.00 mL����4.910 0 g![]() �����������غ㣬�����м����ӵ����ʵ�����
�����������غ㣬�����м����ӵ����ʵ�����![]() �����Ծ���A�Ļ�ѧʽΪK3Fe(C2O4)3��3H2O��
�����Ծ���A�Ļ�ѧʽΪK3Fe(C2O4)3��3H2O��

����Ŀ����ҵ��CO2��CH4�Ⱥ�̼������������Ҫ��Ӧ�á�
��1����ѧ����CH4Ϊԭ�����Ʊ���ϩ��ͬʱ�õ���������֪������ʵı�ȼ�������±���ʾ��д�������Ʊ���ϩ���Ȼ�ѧ����ʽ��__��
���� | ��ȼ����/kJ��mol-1 |
���� | -285.8 |
���� | -890.3 |
��ϩ | -1411.0 |
��2����400��ʱ�����ʼ���Ϊ1L�ĺ����ܱշ�Ӧ���г���1molCH4������������Ӧ�����ƽ����������C2H4���������Ϊ20.0%��
�ٸ��¶��£�ƽ�ⳣ��K=__��
�������ƽ����ϵ�г�������ʵ�����CH4��H2����ƽ�⽫___�����������ƶ������������ƶ����������ƶ���������ȷ��������������__��
��3����ѧ���õ����ز�����ͭ��װ����ͼ��ʾ���˹����ϵͳʵ��CO2�������á�
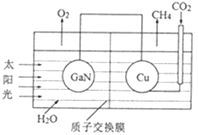
�ٸõ绯ѧװ������__������ԭ�������������������
�ڸõ�ص�Cu�缫�Ϸ�����Ӧ�ĵ缫����ʽΪ__��
����Ŀ���ش��������⣺
(1)��̬̼ԭ�ӵĺ�������Ų�ʽΪ______���ǽ���Ԫ��![]() �ĵ�һ�����ܴ���
�ĵ�һ�����ܴ���![]() �ĵ�һ�����ܣ�ԭ����______��
�ĵ�һ�����ܣ�ԭ����______��
(2)�±��ǵ������ڲ���Ԫ�صĵ�����[��λ��[![]() (���ӷ���)]���ݡ�
(���ӷ���)]���ݡ�
Ԫ�� |
|
|
|
�� | 5.7 | 47.4 | 71.8 |
�� | 7.7 | 15.1 | 80.3 |
�� | 13.0 | 23.9 | 40.0 |
�� | 15.7 | 27.6 | 40.7 |
����˵����ȷ����______(�����)��
A.�Ľ����Ա���ǿ
B.����![]() ��
��
C.��������Ϊ�ǽ���Ԫ��
D.��һ��Ϊ����Ԫ��
(3)![]() ��
��![]() ��Ϊ�������ڹ��ɽ���Ԫ�أ���Ԫ�صIJ��ֵ��������������±���
��Ϊ�������ڹ��ɽ���Ԫ�أ���Ԫ�صIJ��ֵ��������������±���
Ԫ�� |
|
| |
������/( | I1 | 717 | 759 |
I2 | 1509 | 1561 | |
I3 | 3248 | 2957 | |
��Ԫ��λ�ڵ������ڵڢ�B�塣��д����̬![]() �ļ۵����Ų�ʽ��______���Ƚ���Ԫ�ص�I2��I3��֪����̬
�ļ۵����Ų�ʽ��______���Ƚ���Ԫ�ص�I2��I3��֪����̬![]() ��ʧȥ1�����ӱ���̬
��ʧȥ1�����ӱ���̬![]() ��ʧȥ1�������ѣ��Դ���Ľ�����______��
��ʧȥ1�������ѣ��Դ���Ľ�����______��
(4)±��Ԫ��![]() ��
��![]() ��
��![]() ��
��![]() �ĵ縺����С�����˳����______��
�ĵ縺����С�����˳����______��
(5)��̬![]() ԭ�ӵĵ����Ų�ʽΪ______��
ԭ�ӵĵ����Ų�ʽΪ______��![]() ��
��![]() ��ȣ��縺�Խϴ����______��
��ȣ��縺�Խϴ����______��![]() ��
��![]() Ԫ�صĻ��ϼ�Ϊ______��
Ԫ�صĻ��ϼ�Ϊ______��