��Ŀ����
4��������ͨ���ǻ�ɫ��ĩ����ʱ����ɫ���壬��Ҫ������ȡ�����ܵ�ԭ�ϣ���ȡ�Ľ�����������������Ӳ�ʺϽ��ܴźϽ𣬾�����������������������������﮵�ص��������ϣ��ӷ����л��������ܣ�CoO���Ĺ����������£�
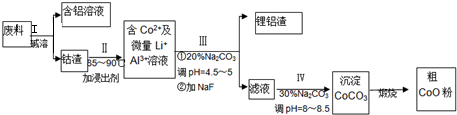
��1�����̢��в���NaOH��Һ�ܳ������е�Al����Ӧ�����ӷ���ʽΪ2Al+2OH-+2H2O=2AlO2-+3H2����
��2���ú�����Һ��ȡAl��OH��3�ҳɱ���ͣ������������������C������ţ�
A������ B���������� C��������̼ D����ˮ
��3�������е�����Co2O3•CoO��ʽ���ڣ��ڹ���II�м���ϡH2SO4�ữ���ټ���Na2S2O3��Һ�����ܣ�������ܵĻ�ѧ����ʽΪ��������ֻ��һ�������4Co2O3•CoO+S2O3 2-+22H+=12Co2++2SO42-+11H2O����ʵ����ģ�ҵ����ʱ��Ҳ������������ܣ���ʵ��������ҵ�в������ᣬ������������ܵ���Ҫԭ����Co2O3•CoO�������������Cl2����Ⱦ������
��4������III�õ����������Ҫ�ɷ������֣�һ����������������һ�ֳɷ���Li2CO3��д��ѧʽ����
��5������III��IV���õ���̼������Һ����д������III����IV��̼������Һ�μӷ�Ӧ�����ַ���ʽ��
�ٹ���III�����ַ���ʽ2Al3++3CO32-+3H2O=2Al��OH��3��+3CO2����2Li++CO32-=Li2CO3����
�ڹ���IV�����ַ���ʽCO32-+2H+=CO2��+H2O��CO32-+Co2+=CoCO3����
���� �Ʊ�����Ϊ�������ü�Һ�ܽ⣬���˵õ�ƫ��������Һ���������������ܽ�������������Ӧ��4Co3O4+Na2S2O3+11H2SO4=12CoSO4+Na2SO4+11H2O���õ����������ӵ���Һ��Ȼ�������ҺpH����̼������Һ��ȥ���������ӣ�ͬʱ̼�������������ӽ������̼��﮳��������ˣ��õ��ϴ����ĺ��������ӵ���Һ���ټ���̼���ơ�������Һ��pH��������ת����̼���ܳ������������̼���ܵõ������ܣ�
��1�������������Ʒ�Ӧ����ƫ�����ƺ�������ע��÷�Ӧ��ˮ�Ƿ�Ӧ�
��2��ƫ������������ᷴӦ����������������������������ǿ�
��3��Co3O4�������������������������·���������ԭ��Ӧ������������ӡ����������Ӻ�ˮ��������л�ԭ�ԣ��ܱ�Co2O3•CoO���������ж���������
��4��̼�������������ӽ������̼��﮳�����
��5���ٹ���III������������̼������ӷ���˫ˮ���������������Ͷ�����̼��̼�������������ӽ������̼��﮳�����
�ڹ���IV��̼�����������ӷ�Ӧ���ɶ�����̼��ˮ��CO32-��Co2+�γ�CoCO3������
��� �⣺�Ʊ������ܵĴ�������Ϊ���Ʊ�����Ϊ�������ü�Һ�ܽ⣬���˵õ�ƫ��������Һ���������������ܽ�������������Ӧ��4Co3O4+Na2S2O3+11H2SO4=12CoSO4+Na2SO4+11H2O���õ����������ӵ���Һ��Ȼ�������ҺpH����̼������Һ��ȥ���������ӣ��õ��ϴ����ĺ��������ӵ���Һ���ټ���̼���ơ�������Һ��pH��������ת����̼���ܳ������������̼���ܵõ������ܣ�
��1����������������Һ��Ӧ����ƫ�����ƺ���������Ӧ�����ӷ�Ӧ����ʽΪ2Al+2OH-+2H2O=2AlO2-+3H2�����ʴ�Ϊ��2Al+2OH-+2H2O=+2AlO2-+3H2����
��2��ƫ������������ᷴӦ����������������������������ǿ�ᣬ���Բ���ѡ�����ᣬѡ�ö�����̼���ʴ�Ϊ��C��
��3��Co3O4��Na2S2O3�����������·���������ԭ��Ӧ����CoSO4��Na2SO4��H2O����Ӧ����ʽΪ��4Co3O4+Na2S2O3+11H2SO4=12CoSO4+Na2SO4+11H2O����д�����ӷ���ʽΪ4Co2O3•CoO+S2O3 2-+22H+=12Co2++2SO42-+11H2O��
������л�ԭ�ԣ��ܱ�Co2O3•CoO���������ж�����������Ⱦ���������Բ������ᣬ
�ʴ�Ϊ��4Co2O3•CoO+S2O3 2-+22H+=12Co2++2SO42-+11H2O��Co2O3•CoO�������������Cl2��Ⱦ������
��4��̼�������������ӽ������̼��﮳����������III�õ����������Ҫ�ɷ������֣�һ����������������һ�ֳɷ���Li2CO3���ʴ�Ϊ��Li2CO3��
��5���ٹ���III������������̼������ӷ���˫ˮ���������������Ͷ�����̼���䷴Ӧ�ķ���ʽΪ2Al3++3CO32-+3H2O=2Al��OH��3��+3CO2����̼�������������ӽ������̼��﮳������䷴Ӧ�ķ���ʽΪ2Li++CO32-=Li2CO3����
�ʴ�Ϊ��2Al3++3CO32-+3H2O=2Al��OH��3��+3CO2����2Li++CO32-=Li2CO3����
�ڹ���IV��̼�����������ӷ�Ӧ���ɶ�����̼��ˮ���䷴ӦΪCO32-+2H+=CO2��+H2O��CO32-��Co2+�γ�CoCO3���������䷴ӦΪCO32-+Co2+=CoCO3����
�ʴ�Ϊ��CO32-+2H+=CO2��+H2O��CO32-+Co2+=CoCO3����
���� ���⿼�������ʵķ����ᴿ�����Ͳ������ۺ�Ӧ�ã�����������ԭ��Ӧ�����ӷ�Ӧ����ϵʽ����ȣ���Ŀ�Ѷ��еȣ����ؼ�����ʵ����������������ԭ��Ӧ�жϷ��������ӷ�Ӧ�����ض�ѧ���ۺ������Ŀ��飬��һ�������Ϻõ����������⣮

| A�� | 0.1mol•L-1�ģ�NH4��2Fe��SO4��2��Һ�У�c��Fe2+��+c��NH4+��+c��H+��=c��SO42-��+c��OH-�� | |
| B�� | ����������ʵ���Ũ�ȵ�Na2CO3��NaHCO3��Һ��ϣ�2c��CO32-��+2c��HCO3-��+2c��H2CO3��=3c��Na+�� | |
| C�� | 0.2mol•L-1��CH3COOH��Һ��0.1mol•L-1��NaOH��Һ�������ϣ�c��CH3COOH��+2c��H+��=c��CH3COO-��+2c��OH-�� | |
| D�� | PH��ͬ��CH3COONa��Һ��NaHCO3��Һ��NaOH��Һ��c��NaHCO3����c��CH3COONa����c��NaOH�� |
| A�� | ����Һ�ж����ڣ�c��H+��=c��CH3COO-��+c��OH-�� | |
| B�� | ����Һ����ˮ�������c��H+���ֱ���1.0��10-11mol/L��1.0��10-9mol/L | |
| C�� | ����Һ�зֱ��������ˮϡ��100��ʱ����Һ��c��H+��֮�ȴ���100��1 | |
| D�� | �����������Һ�ֱ�����ͬŨ�ȵ�NaOH��Һ��ȫ�����кͷ�Ӧʱ�������NaOH��Һ�����Ϊ1��100 |
| A�� | ����һ�ֵ���ɫ��������ˮ�ľ��壬�������������л�ԭ�� | |
| B�� | NO2����ˮ�������ᣬ����NO2������������ | |
| C�� | Cl2���ڳ�������������˳����д����������Ӧ | |
| D�� | ����Ӧ�ù㷺�İ뵼����ϣ������»�ѧ���ʲ����ã�����Ȼ����������̬���� |
��1���������б�������ʵ��������������Ԫ�صı߽磮
��3��������ĽṹʽΪ
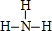 �����ʱ��ĵ���ʽΪ
�����ʱ��ĵ���ʽΪ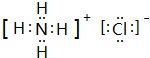 ��
����4��Ԫ��B��D��ɵĻ����ﶡ����ɫֲ����й�����õ���Ҫԭ��֮һ���õ���ʽ��ʾ�����ﶡ���γɹ��̣�
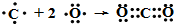 ��
����5��Ԫ��D�ļ�������Ԫ��E�ļ����ӵİ뾶��С��ϵ��O2-��Al3+�������ӷ��ű�ʾ����
��6��B��C��F������������Ӧˮ�����������ǿ������˳����HClO4��HNO3��H2CO3���û�ѧʽ��ʾ����
��7��д��Ԫ��E�������������Ԫ��C������������Ӧˮ���ﷴӦ�����ӷ���ʽAl2O3+6H+=2Al3++3H2O��
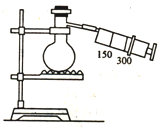 ��ͼ��ʾ��ͼ�в�������δ��������һ�ݻ�Ϊ300mL��ע��������������ƿ��500mL�������ӣ���ƿ����0.384g CuƬ��������ƿ�м���18mL 2.5mol•L-1��ϡHNO3��Һ����������������ס��Ƥ����סƿ�ڣ������������Ϊ��״���µģ��Իش�
��ͼ��ʾ��ͼ�в�������δ��������һ�ݻ�Ϊ300mL��ע��������������ƿ��500mL�������ӣ���ƿ����0.384g CuƬ��������ƿ�м���18mL 2.5mol•L-1��ϡHNO3��Һ����������������ס��Ƥ����סƿ�ڣ������������Ϊ��״���µģ��Իش�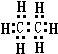 ������ʽΪCmH20��������mΪ9������ʽΪC8Hn��������nΪ18����CO2 �ܶȣ�ͬ��ͬѹ����ͬ����������ʽΪC3H8����������������Ϊ1mol��������O2��ȼ�գ�����O2������C9H20��
������ʽΪCmH20��������mΪ9������ʽΪC8Hn��������nΪ18����CO2 �ܶȣ�ͬ��ͬѹ����ͬ����������ʽΪC3H8����������������Ϊ1mol��������O2��ȼ�գ�����O2������C9H20��