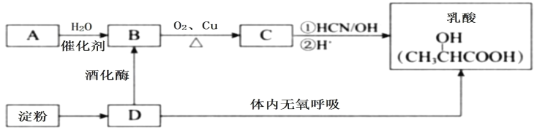
��Ŀ����
����Ŀ������ʪ���Ʊ������һ�ֹ����������£�

��֪��������Ҫ�ɷ�ΪCa5(PO4)3(OH)��������Ca5(PO4)3F���л�̼�ȡ��ܽ�ȣ�Ca5(PO4)3(OH)<CaSO4��0.5H2O
��1�������������ܼӿ췴Ӧ���ʵĴ�ʩ��___��
��2����������ʱ������Ӧ��2Ca5(PO4)3(OH)+3H2O+10H2SO4![]() 10CaSO4��0.5H2O+6H3PO4
10CaSO4��0.5H2O+6H3PO4
�ٸ÷�Ӧ���ֳ����Թ�ϵ��H3PO4___H2SO4���>����<������
�ڽ��Ԫ�������ɽ��͢��н��ۣ�P��S���Ӳ�����ͬ��___��
��3�����ʱ��������Ca5(PO4)3F������ת��ΪHF������һ��ת��ΪSiF4��ȥ��д������HF�Ļ�ѧ����ʽ��__��
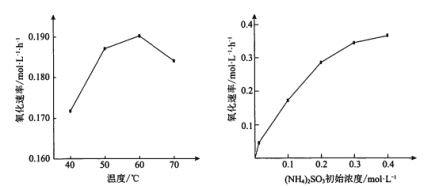
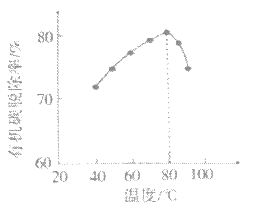
��4��H2O2���������е��л�̼����ΪCO2�ѳ���ͬʱ����Ҳ�ᷢ���ֽ⡣��ͬͶ�ϱȡ���ͬ��Ӧʱ�䣬��ͬ�¶��µ��л�̼�ѳ�����ͼ��ʾ��80����ѳ��ʱ仯��ԭ��___��
��5������ʱ��CaCO3�Թ�������ַ�Ӧ������SO42������������BaCO3�ɽ�һ���������ѳ��ʣ������ӷ���ʽ��__��
���𰸡���ĥ������ < �˵����P<S��ԭ�Ӱ뾶P>S���õ�������P<S���ǽ�����P<S 2Ca5(PO4)3F+10H2SO4+5H2O��10CaSO4��0.5H2O+6H3PO4+2HF�� 80���H2O2�ֽ����ʴ�Ũ���������� BaCO3+SO42��+2H3PO4��BaSO4+CO2��+H2O+2H2PO4��
��������
(1)���üӿ컯ѧ��Ӧ���ʵĴ�ʩ����ĥ�����ȣ��ܽ�ʱ����ȣ�
(2)�ٸ��ݷ�Ӧ����ʽ������H2SO4�μӷ�Ӧ�õ�H3PO4������ǿ����ȡ���
��H3PO4��H2SO4��Ϊ��ۺ����ᣬ��Ҫ�ɴ�P��S�ķǽ����ԽǶȿ��ǣ�S�ķǽ�����ǿ��P������ʹO�ϵ������ܶȽ����̶�����H+�����룬H+Խ���룬����������Խǿ��
(3)���ʱ��������Ca5(PO4)3F������ת��ΪHF����Ӧ����ΪCa5(PO4)3F��H2SO4��Ӧ������CaSO4![]() H2O��H3PO4��HF���ݴ�д����Ӧ����ʽ��
H2O��H3PO4��HF���ݴ�д����Ӧ����ʽ��
(4)����ͼ��80��ǰ�����¶����ߣ��л�̼�ѳ�������80��������¶����ߣ��л�̼�ѳ��ʽ��ͣ�����H2O2���ȷֽ⣬����H2O2Ũ�Ƚ���Ӱ���л�̼���ѳ��ʣ�
(5)��������Ǽ���CaCO3������Ӧ������ʱ��CaCO3�Թ��������������ᷴӦ����CaSO4����CaSO4�ܣ����Գ�ַ�Ӧ������SO42-����������BaCO3�ɽ�һ���������ѳ��ʣ�����BaCO3�μӷ�Ӧת���������ܵ�BaSO4���ڳ���ת���ķ�Ӧ����֮�Ϸ�����һ����Ӧ��ʹ������г̶����ݴ˷�����
(1)���üӿ컯ѧ��Ӧ���ʵĴ�ʩ����ĥ�����ȣ��ܽ�ʱ����ȡ��ʴ�Ϊ����ĥ�����ȣ�
(2)�ٸ��ݷ�Ӧ����ʽ������H2SO4�μӷ�Ӧ�õ�H3PO4������ǿ����ȡ���ᣬ�������ǿ��Ϊ��H3PO4��H2SO4��
��H3PO4��H2SO4��Ϊ��ۺ����ᣬ��Ҫ�ɴ�P��S�ķǽ����ԽǶȿ��ǣ�S�ķǽ�����ǿ��P������ʹO�ϵ������ܶȽ����̶�����H+�����룬H+Խ���룬����������Խǿ�����Լ���Ϊ��P�İ뾶����S��P�ķǽ�����С��S������H3PO4������С��H2SO4�����ԣ�
(3)���ʱ��������Ca5(PO4)3F������ת��ΪHF����Ӧ����ΪCa5(PO4)3F��H2SO4��Ӧ������CaSO4![]() H2O��H3PO4��HF�����Ի�ѧ��Ӧ����ʽΪ��2Ca5(PO4)3F+10H2SO4+5H2O
H2O��H3PO4��HF�����Ի�ѧ��Ӧ����ʽΪ��2Ca5(PO4)3F+10H2SO4+5H2O ![]() 10CaSO4
10CaSO4![]() H2O+6H3PO4+2HF��
H2O+6H3PO4+2HF��
(4)����ͼ��80��ǰ�����¶����ߣ��л�̼�ѳ�������80��������¶����ߣ��л�̼�ѳ��ʽ��ͣ�����H2O2���ȷֽ⣬����H2O2Ũ�Ƚ���Ӱ���л�̼���ѳ��ʣ����Կ��Խ���Ϊ���¶ȸ���80��ʱ��H2O2�ķֽ����ʼӿ죬����H2O2��Ũ�Ƚ��ͣ�Ҳ�͵����л�̼�ѳ����½���
(5)����BaCO3�ɽ�һ���������ѳ��ʣ�����BaCO3�μӷ�Ӧת���������ܵ�BaSO4��BaSO4�����CaSO4�ܽ�ȸ��ͣ���ʹ��Һ��SO42-Ũ�ȸ��ͣ����ǵ�����ת���ķ�ӦЧ�ʽϵͣ����Խ�һ���������ѳ������ǿ����ڳ���ת����Ӧ�Ļ����Ϸ���Ч�ʸ��ߵķ�Ӧ����ʹ������Ӧ�Ľ��У�����ת����ӦΪ��BaCO3+SO42-![]() BaSO4+CO32-����ʱ��Һ���������һ�������ԣ����Լ���������Ӧ����CO2������������Ӧ��������������еij̶ȸ����Դﵽ��һ���ѳ��ʵ�Ч�����ۺϿ��������ӷ���ʽΪ��BaCO3+SO42-+H3PO4�TBSO4+CO2��+H2O+H2PO4-��
BaSO4+CO32-����ʱ��Һ���������һ�������ԣ����Լ���������Ӧ����CO2������������Ӧ��������������еij̶ȸ����Դﵽ��һ���ѳ��ʵ�Ч�����ۺϿ��������ӷ���ʽΪ��BaCO3+SO42-+H3PO4�TBSO4+CO2��+H2O+H2PO4-��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ����������������Ҫ�ɷ�ΪFe2O3��Fe3O4���Լ�����SiO2��Al2O3�ȡ��������������Ʊ����죨Fe2O3����һ�ֹ����������£�

��֪����ԭ����ʱ����Fe2O3��Fe3O4ת��ΪFeO��
�������ӿ�ʼ��������ȫ����ʱ��pH���±���ʾ��
���� | ��ʼ����ʱ��pH | ��ȫ����ʱ��pH |
Fe2+ | 7.6 | 9.7 |
Fe3+ | 2.7 | 3.7 |
Al3+ | 3.8 | 4.7 |
��1���������������������������Һ�еĽ����������У������ӷ��ţ�________________��
��2��Fe�۳���pH�⣬��һ��������___________��Fe�۵�����Һ��pHΪ__________��
��3��������������������������FeCO3�����ӷ���ʽΪ_______________________________��
������Һ����Ҫ�����ǣ��ѧʽ��_______________��
��4�����������£��������������з�����Ӧ�Ļ�ѧ����ʽΪ______________________________��
����Ŀ��X��Y��Z��WΪ��ԭ��������С�������е����ֶ�����Ԫ�أ���֪��![]() Ԫ��ԭ�Ӽ۵����Ų�ʽΪ
Ԫ��ԭ�Ӽ۵����Ų�ʽΪ![]() ����ԭ�Ӱ뾶��ͬ��Ԫ������С�ġ�
����ԭ�Ӱ뾶��ͬ��Ԫ������С�ġ�![]() Ԫ���ǵؿ��к�������Ԫ�أ�WԪ�صĵ縺����С��YԪ�أ���Wԭ�ӵĵ����Ų��У�p�����ֻ��һ��δ�ɶԵ��ӡ�
Ԫ���ǵؿ��к�������Ԫ�أ�WԪ�صĵ縺����С��YԪ�أ���Wԭ�ӵĵ����Ų��У�p�����ֻ��һ��δ�ɶԵ��ӡ�![]() Ԫ�صĵ������������
Ԫ�صĵ������������![]() ��
��
|
|
|
|
|
496 | 4562 | 6912 | 9540 |
|
��ش�
![]() �ĵ���ʽΪ______�����еĻ�ѧ������Ϊ______��
�ĵ���ʽΪ______�����еĻ�ѧ������Ϊ______��![]() Ϊ______���塣
Ϊ______���塣
![]() ��ˮ����ǿ��ˮ�����һ������A����Һ�ʼ��ԣ���A�ĽṹʽΪ______������ӿռ乹��Ϊ______��
��ˮ����ǿ��ˮ�����һ������A����Һ�ʼ��ԣ���A�ĽṹʽΪ______������ӿռ乹��Ϊ______��
![]() ��Y��Z��W����Ԫ�����γɵĵ����У�Ӳ��������______
��Y��Z��W����Ԫ�����γɵĵ����У�Ӳ��������______![]() �����ʵ�����
�����ʵ�����![]() ������
������![]() ���Ըߵ�ԭ����______��
���Ըߵ�ԭ����______��![]() ��Xԭ�ӵ��ӻ��������Ϊ______��
��Xԭ�ӵ��ӻ��������Ϊ______��
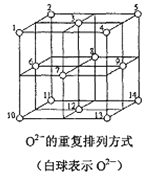
![]() ����Ľṹʾ��ͼ��ͼ��ʾ����þ����Ħ������Ϊ
����Ľṹʾ��ͼ��ͼ��ʾ����þ����Ħ������Ϊ![]() ��������ܶ�Ϊ
��������ܶ�Ϊ![]() �����ӵ�����Ϊ
�����ӵ�����Ϊ![]() �����������������Z�������ļ�ľ���
�����������������Z�������ļ�ľ���![]() ______��
______��