��Ŀ����
����Ŀ��Na2S2O3����Ҫ�Ļ���ԭ�ϣ�������ˮ�������Ի���Ի������ȶ���
���Ʊ�Na2S2O35H2O��Ӧԭ����Na2SO3(aq)��S(s)![]() Na2S2O3(aq)
Na2S2O3(aq)
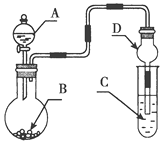
ʵ�鲽�裺
�ٳ�ȡ15 g Na2SO3����Բ����ƿ�У��ټ���80 mL����ˮ����ȡ5 g��ϸ����ۣ���3 mL�Ҵ���ʪ������������Һ�С�
�ڰ�װʵ��װ��(��ͼ��ʾ�����ּг�װ����ȥ)��ˮԡ���ȣ���60 min��
�۳��ȹ��ˣ�����Һˮԡ����Ũ������ȴ����Na2S2O35H2O�������ˡ�ϴ�ӡ�����õ���Ʒ��
�ش����⣺
(1)����a��������_________����������__________________________��
(2)��Ʒ�г�����δ��Ӧ��Na2SO3�⣬����ܴ��ڵ���������________�������Ƿ���ڸ����ʵķ�����________________________________��
(3)��ʵ��һ������ڼ��Ի����½��У������Ʒ���ƣ������ӷ�Ӧ����ʽ��ʾ��ԭ��________��
�ⶨ��Ʒ����
ȷ��ȡW g��Ʒ������������ˮ�ܽ⣬�Ե�����ָʾ������0.100 0 molL-1��ı���Һ�ζ�����Ӧԭ��Ϊ2S2O32-��I2��S4O62-��2I-��
(4)�ζ����յ�ʱ���ж�������___________________________��
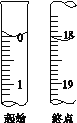
(5)�ζ���ʼ���յ��Һ��λ����ͼ�������ĵ�ı���Һ���Ϊ_______mL����Ʒ�Ĵ���Ϊ(��Na2S2O35H2O��Է�������ΪM)_________��(�ú�M��ʽ�ӱ���)
���𰸡����������� �������� Na2SO4 ȡ������Ʒ���ڹ������ᣬ���ˣ�����Һ�м�BaCl2��Һ�����а�ɫ���������Ʒ�к���Na2SO4 S2O32-��2H��= S����SO2����H2O �������һ�α�Һ����Һ��ɫ����ɫ����ɫ��������ڲ���ȥ 18.10 ![]() ��100%
��100%
��������
��1����������ͼʾװ��ͼ��֪������aΪ���������ܣ���ʵ�������������ܾ�����������ˮ���Ҵ������ã�
��2��S2O32-���л�ԭ�ԣ��ܹ���������������������ӣ����Կ��ܴ��ڵ������������ƣ����������Ƶķ���Ϊ��ȡ������Ʒ���ڹ���ϡ���ᣬ���ˣ�����Һ�м�BaCl2��Һ�����а�ɫ���������Ʒ�к���Na2SO4��
��3��S2O32-�������ӷ���������ԭ��Ӧ���ɵ���ɫ���ʣ���Ӧ�����ӷ���ʽΪ��S2O32-+2H��=S��+SO2��+H2O��
��4���ζ������ⵥ��ʹ���۱��������Եζ����յ�ʱ���ж������ǵ������һ�α�Һ����Һ��ɫ����ɫ����ɫ��������ڲ���ȥ��
��5������ͼʾ�ĵζ�����Һ���֪���ζ����г�ʼ����Ϊ0���ζ��յ�Һ�����Ϊ18.10mL���������ĵ�ı���Һ���Ϊ18.10mL�����ݷ�Ӧ2S2O32-+I2�TS4O62-+2I-��֪��n(S2O32-)=2n(I2)������W g��Ʒ�к���Na2S2O3��5H2O����Ϊ��0.1000 mol��L-1��18.10��10-3L��2��M=3.620��10-3M g�����Ʒ�Ĵ���Ϊ��![]() ��100%��
��100%��

����Ŀ������K2Cr2O7�ķ�ˮ���н�ǿ�Ķ��ԣ���ҵ�ϳ��ñ��γ�������������K2Cr2O7�ķ�ˮ�������ظ��ᣬ������������£�

��֪��CaCr2O7��BaCr2O7������ˮ�������������ڳ����µ��ܶȻ��������±���ʾ��
���� | CaSO4 | CaCrO4 | BaCrO4 | BaSO4 |
�ܶȻ� | 9.1��10-6 | 2.30��10-2 | 1.17��10-10 | 1.08��10-10 |
��1�������ӷ���ʽ��ʾK2Cr2O7��Һ��ͬʱ����K2CrO4��ԭ�����ӷ���ʽ������������
![]() +__________=
+__________=![]() +__________��____________
+__________��____________
��2������Һ1�м���BaCl2��2H2O��Ŀ�ģ���ʹ![]() ����Һ�г���������
����Һ�г���������
�ٽ����������˵����ʯ�ҵ����ã�__________��
�ڽ�ϱ������ݣ�˵��ѡ��Ba2+����ѡ��Ca2+������ˮ�����ɣ�__________��
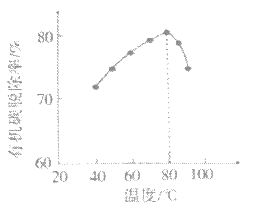
���о��¶ȶ�![]() ����Ч�ʵ�Ӱ�졣ʵ�������£�����ͬ��ʱ�����ڣ���ͬ�¶���
����Ч�ʵ�Ӱ�졣ʵ�������£�����ͬ��ʱ�����ڣ���ͬ�¶���![]() �ij�����
�ij����� ������ͼ��ʾ��
������ͼ��ʾ��

��֪��BaCrO4(s) ![]() Ba2+(aq)+
Ba2+(aq)+ ![]() ��H>0
��H>0
![]() �ij���Ч�����¶ȱ仯��ԭ����__________��
�ij���Ч�����¶ȱ仯��ԭ����__________��
��3�������2�м������ᣬ�����ظ��ᡣ
������Ũ�ȶ��ظ���Ļ���������ͼ��ʾ����ϻ�ѧƽ���ƶ�ԭ��������ʹ��0.450mol/L������ʱ���ظ���Ļ��������Ը���ʹ��0.225mol/L�������ԭ��__________��

�ڻ����ظ����ԭ����ͼ��ʾ��������Ũ�ȸ���0.450mol/Lʱ���ظ���Ļ�����û�����Ա仯����ԭ����__________��

��4����������������BaCrO4����һ�������ظ����Ч����__________�йء�
���𰸡� 1 H2O 2 2 H+ ����![]() ��������ҺpH��ʹ
��������ҺpH��ʹ![]() ת��Ϊ
ת��Ϊ![]() ������ BaCrO4��CaCrO4�����ܣ�����ʹ
������ BaCrO4��CaCrO4�����ܣ�����ʹ![]() ��������ȫ �¶����ߣ��������ʼӿ� c(H2SO4)������c(
��������ȫ �¶����ߣ��������ʼӿ� c(H2SO4)������c(![]() )������Ba2+���ɳ������ٽ�BaCrO4(s)
)������Ba2+���ɳ������ٽ�BaCrO4(s) ![]() Ba2+(aq)+
Ba2+(aq)+ ![]() (aq)��H>0ƽ�����ƣ�c(
(aq)��H>0ƽ�����ƣ�c(![]() )����ͬʱ��c(H+)Ҳ����ͬ�ٽ�
)����ͬʱ��c(H+)Ҳ����ͬ�ٽ�![]() +H2O
+H2O![]()
![]() +2H+ƽ�����ƣ����������ɸ����H2Cr2O7 BaSO4��BaCrO4�ܽ�Ƚӽ���c(H2SO4)Խ��Խ����������BaSO4��������BaCrO4�⣬ʹ�����ڽӴ�H2SO4���谭�ظ������� �ܵ���ҺpH���¶ȡ�H2SO4Ũ�ȡ�BaCrO4������С��Ӱ��
+2H+ƽ�����ƣ����������ɸ����H2Cr2O7 BaSO4��BaCrO4�ܽ�Ƚӽ���c(H2SO4)Խ��Խ����������BaSO4��������BaCrO4�⣬ʹ�����ڽӴ�H2SO4���谭�ظ������� �ܵ���ҺpH���¶ȡ�H2SO4Ũ�ȡ�BaCrO4������С��Ӱ��
������������K2Cr2O7�ķ�ˮͬʱ����SO42������������ʯ�ҹ��˵õ��Ĺ���1Ϊ����ƺ���Һ1������Һ1�м���BaCl22H2O��Ŀ����ʹCrO42����Һ�г������������˵õ�����2ΪBaCrO4����Һ2���CrO42������꣬�����2�м������ᣬ�����ظ��ᣬͬʱ���ɹ���3Ϊ���ᱵ������
(1). K2Cr2O7��Һ��ͬʱ����K2CrO4��ԭ�����ظ����������ˮ��Һ�д��ڻ�ѧƽ�����ɸ�������Ӻ�����������Ӧ�����ӷ���ʽΪCr2O72+H2O2CrO42+2H+���ʴ�Ϊ��1��H2O��2��2��H+��
(2). ��. ���ݱ������ʵ��ܶȻ�������֪����ʯ�ҵ������dz�����������ӣ�������ҺpHʹCr2O72ת��ΪCrO42���������ʴ�Ϊ������SO42����������ҺpH��ʹCr2O72ת��ΪCrO42��������
��. ���ݱ������ݿ�֪��BaCrO4��CaCrO4�����ܣ�����ѡ��Ba2+������ˮ����ʹCrO42��������ȫ���ʴ�Ϊ��BaCrO4��CaCrO4������������ʹCrO42��������ȫ��
��. ����ͼ���֪��CrO42�ij��������¶��������������ʼӿ죬�ʴ�Ϊ���¶����ߣ��������ʼӿ죻
(3). ��. c(H2SO4)��������c(SO42��)��������Ba2+���ɳ�������ʹ![]() Ba2+(aq)+CrO42(aq)ƽ��������ʹc(CrO42)������ͬʱc(H+)Ҳ��������ͬ��ʹCr2O72+H2O
Ba2+(aq)+CrO42(aq)ƽ��������ʹc(CrO42)������ͬʱc(H+)Ҳ��������ͬ��ʹCr2O72+H2O![]() 2CrO42+2H+ƽ�����ƣ��������ɸ����H2Cr2O7���ʴ�Ϊ��c(H2SO4)��������c(SO42��)��������Ba2+���ɳ�������ʹBaCrO4(s)
2CrO42+2H+ƽ�����ƣ��������ɸ����H2Cr2O7���ʴ�Ϊ��c(H2SO4)��������c(SO42��)��������Ba2+���ɳ�������ʹBaCrO4(s) ![]() Ba2+(aq)+CrO42(aq)ƽ��������c(CrO42)������ͬʱc(H+)Ҳ��������ͬ��ʹCr2O72+H2O
Ba2+(aq)+CrO42(aq)ƽ��������c(CrO42)������ͬʱc(H+)Ҳ��������ͬ��ʹCr2O72+H2O![]() 2CrO42+2H+ƽ�����ƣ��������ɸ����H2Cr2O7��
2CrO42+2H+ƽ�����ƣ��������ɸ����H2Cr2O7��
��. ���ݱ��и����ʵ��ܶȻ�������֪��BaSO4��BaCrO4�ܽ�ȱȽϽӽ���c(H2SO4)Խ����Խ����������BaSO4��������BaCrO4����ʹ������H2SO4�Ӵ����谭�ظ������ɣ��ʴ�Ϊ��BaSO4��BaCrO4�ܽ�Ƚӽ���c(H2SO4)Խ��Խ����������BaSO4��������BaCrO4�⣬ʹ�����ڽӴ�H2SO4���谭�ظ���������
(4). ��������������֪������BaCrO4����һ�������ظ����Ч�����¶���Ũ�ȡ���ҺpH������Ũ���������������й����ܵ���ҺpH���¶ȡ�H2SO4Ũ�ȡ�BaCrO4������С�����ص�Ӱ�죬�ʴ�Ϊ���ܵ���ҺpH���¶ȡ�H2SO4Ũ�ȡ�BaCrO4������С��Ӱ�졣
�����͡�������
��������
19
����Ŀ����Na2SO3��Һ�Ͳ�ͬ��������������Һ��Ϊʵ�����̽���ε����ʺ�����Һ�䷴Ӧ�Ķ����ԡ�
ʵ�� | �Լ� | ���� | |
�ι� | �Թ� | ||
| 0.2mol��L-1Na2SO3��Һ | ����Ag2SO4��Һ | ������ɫ���� |
0.2mol��L-1CuSO4 | ����Һ���̣������μӲ����ػ�ɫ���� | ||
0.1mol��L-1Al2(SO4)3��Һ | ��ʼ�����Ա仯�������μӲ�����ɫ���� | ||
��1������죬������еİ�ɫ������Ag2SO3�������ӷ���ʽ���������__________��
��2�������飬�������ػ�ɫ�����в���![]() ������Cu+��Cu2+��
������Cu+��Cu2+��![]() ��
��
��֪��Cu+![]() Cu+Cu2+��Cu2+
Cu+Cu2+��Cu2+![]() CuI��(��ɫ)+I2��
CuI��(��ɫ)+I2��
����ϡ����֤ʵ�����к���Cu+��ʵ��������__________��
��ͨ������ʵ��֤ʵ�������к���Cu2+��![]() ��
��

a����ɫ����A��BaSO4���Լ�1��__________��
b��֤ʵ�����к���Cu2+��![]() ��������__________��
��������__________��
��3����֪��Al2(SO3)3��ˮ��Һ�в����ڡ������飬�����İ�ɫ��������![]() ���ð�ɫ������������ǿ�ᣬ��������ǿ�����ʹ����KMnO4��Һ��ɫ��
���ð�ɫ������������ǿ�ᣬ��������ǿ�����ʹ����KMnO4��Һ��ɫ��
���Ʋ�����к������������__________��
�ڶ��ڳ�������������Ĵ�����ʽ������ּ��裺
i��Al(OH)3��������
ii�����������ļ�ʽ���С��Լ���ii����˶Ա�ʵ�飬֤ʵ�˼���ii������
a�����Ա�ʵ�鷽������������
����һ��

�������__________������ͼ��ʽ���֣���
b������ii������ʵ��֤����__________��
��4������ʵ�飬�������ε�������__________������Һ�䷴Ӧ�Ķ�������__________�йء�