��Ŀ����
19��Ϊ�˲ⶨij�л���A�Ľṹ��������ʵ�飺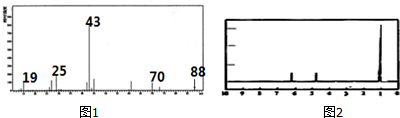
�ٽ�4.4g���л�����ȫȼ�գ����� 0.2mol CO2��3.6gˮ��
���������Dzⶨ����Է�������������ͼ1��ʾ������ͼ��
��A�ĺ˴Ź���������ͼ2��
�Իش��������⣺
��1���л���A����Է���������88��
��2���л���A��ʵ��ʽ��C2H4O��
��3���л���A�ķ���ʽC4H8O2��
��4����������Ϸ���A��-COOH��2��-CH3���л���A�Ľṹ��ʽ��CH3��2CHCOOH��
��5��д��A������ͬ���칹��CH3CH2COOH��CH3COOCH3����HCOOCH2CH3����
���� ��1������ͼ1�е�����ͼ�����жϸ��л������Է���������
��2������n=$\frac{m}{M}$�����4.4g���л��P3.6gˮ�����ʵ�����Ȼ�����������غ�ȷ�������ʽ��Ȼ���ȷ����ʵ��ʽ��
��3�����ݣ�2����֪���л���ķ���ʽ��
��4�����������֪�����л�������к���1��-COOH��2��-CH3����������ʽ��ȷ����ṹ��ʽ��
��5��AΪ��CH3��2CHCOOH����ͬ���칹�����Ϊ����һԪ���ᣬ�п������������ʣ��ݴ�д�������������л����ͬ���칹�壮
��� �⣻��1���������Dzⶨ����Է�������������ͼ1��ʾ������ͼ�����ݸ��л��������ͼ��֪������Է�������Ϊ88��
�ʴ�Ϊ��88��
��2��4.4g���л�������ʵ���Ϊ��$\frac{4.4g}{88g/mol}$=0.05mol��3.6gˮ�����ʵ���Ϊ��$\frac{3.6g}{18g/mol}$=0.2mol��
���������غ㣬���л�������к���C��Hԭ����Ϊ��N��C��=$\frac{0.2mol}{0.05mol}$=4��N��H��=$\frac{0.2mol��2}{0.05mol}$=8��������к���C��H����ԭ����Ϊ��12��4+1��8=56��˵�����л�������л�����OԪ�أ�������Ԫ�صĸ���Ϊ��$\frac{88-56}{16}$=2�����Ը��л���ķ���ʽΪ��C4H8O2����ʵ��ʽΪ��C2H4O��
�ʴ�Ϊ��C2H4O��
��3�����ݣ�2����֪�����л���ķ���ʽΪ��C4H8O2��
�ʴ�Ϊ��C4H8O2��
��4����������Ϸ���A��-COOH��2��-CH3����ϸ��л������ʽC4H8O2��֪����ṹ��ʽΪ����CH3��2CHCOOH��
�ʴ�Ϊ����CH3��2CHCOOH��
��5��AΪ��CH3��2CHCOOH�������ʽΪC4H8O2����ͬ���칹�����Ϊ����һԪ���ᣬ�п���Ϊ�������ʣ�����������л����У�CH3CH2COOH��CH3COOCH3��HCOOCH2CH3��
�ʴ�Ϊ��CH3CH2COOH��CH3COOCH3����HCOOCH2CH3����
���� ���⿼�����л������ʽ���ṹ��ʽ��ȷ������Ŀ�Ѷ��еȣ���ȷ����ͼ���˴Ź������ĺ���Ϊ���ؼ���ע�����������غ㶨����ȷ���л������ʽ�е�Ӧ�÷���������֪ʶ��϶࣬�ۺ��Խ�ǿ����ֿ���ѧ���ķ������������������Ӧ�û���֪ʶ��������

 ����������ϵ�д�
����������ϵ�д�| ��� | ���������� | ���� |
| A | ��������NaOH�Ҵ���Һ���Ȳ���������ͨ��KMnO4������Һ�У���Һ��ɫ | ����������Ϊ��ϩ |
| B | ��0.1mol•L-1��ˮϡ�ͳ�0.01mol•L-1�����pH��11.1��� 10.6 | ϡ�ͺ�NH3•H2O�ĵ���̶ȼ�С |
| C | �����£���ñ���Na2CO3��Һ��pH���ڱ���NaHCO3��Һ | ������ˮ��̶ȣ�CO32-��HCO3- |
| D | ��25mL��ˮ�ͷ�ˮ�зֱ����5��FeCl3������Һ��ǰ��Ϊ��ɫ������Ϊ���ɫ | �¶����ߣ�Fe3+��ˮ��̶����� |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | Na��Mg��Alԭ�������������������� | |
| B�� | P��S��ClԪ����������������� | |
| C�� | N��O��F��һ�������������� | |
| D�� | Na��K��Rb�縺����С |
| A�� | -CHO�ĵ���ʽ�� | B�� | ������ӵı���ģ��Ϊ�� | ||
| C�� | ˳-1��2-������ϩ�ṹʽΪ�� | D�� | 1��4-���ױ��Ľṹ��ʽΪ�� |
| A�� | ���ڱ��е�VA��Ԫ�ص�����������Ӧˮ����Ļ�ѧʽ��ΪH3RO4 | |
| B�� | O${\;}_{2}^{2-}$��S2-������ͬ���������͵����� | |
| C�� | 뭡�뮡�밷ֱ���Oԭ���γɵ�ˮH2O��D2O��T2O�Ļ����ʲ�ͬ | |
| D�� | �γ����Ӽ����������Ӽ�ֻ���ھ��������� |

 ��A��F
��A��F ��
��
 ����B��
����B�� ��
��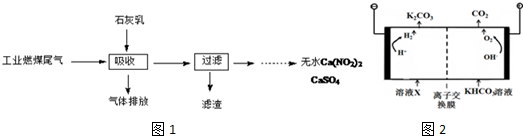
 H2SO3��2H2SO3+O2$\frac{\underline{\;����\;}}{\;}$2H2SO4��
H2SO3��2H2SO3+O2$\frac{\underline{\;����\;}}{\;}$2H2SO4��