��Ŀ����
20��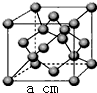 ��aX��bY��cZ����Ԫ�أ���֪���ٸ�ԭ������a��b��c��С��20��a+b+c=25����Ԫ��Y�ĵ�����Χ���ӹ���Ϊnsnnpn+2����X��Y�ڲ�ͬ�����¿��γ�X2Y��X2Y2���ֻ����Y��Z�ڲ�ͬ�����¿��γ�ZY��ZY2���ֻ������Z���������Է���������Z���Ȼ������Է�������֮��Ϊ38��77�����Ͽ���֪��
��aX��bY��cZ����Ԫ�أ���֪���ٸ�ԭ������a��b��c��С��20��a+b+c=25����Ԫ��Y�ĵ�����Χ���ӹ���Ϊnsnnpn+2����X��Y�ڲ�ͬ�����¿��γ�X2Y��X2Y2���ֻ����Y��Z�ڲ�ͬ�����¿��γ�ZY��ZY2���ֻ������Z���������Է���������Z���Ȼ������Է�������֮��Ϊ38��77�����Ͽ���֪����1��X��Na��Y��O��Z��C��дԪ�ط��ţ���
��2��X2Y2�����Ӿ��壬���ɾ��������Na+��O22-���þ����к������Ӽ������ۼ������������ã���
��3��Z��������Ȼ���ķ��ӿռ乹�ͷֱ���ֱ���Ρ��������壬����Zԭ�ӷֱ���sp��sp3�ӻ�����ɼ������ݵ������ص���ʽ��Z����������к��еļ������༰��Ŀ��2���Ҽ���2���м���
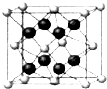
��4��Z��һ�ֵ��ʵľ����ṹ����ͼ��ʾ������Zԭ�ӵ���λ��Ϊ4�������ܶȦ�=$\frac{96}{{a}^{3}•{N}_{A}}$g��cm-3����ֻд����ʽ��
���� Ԫ��Y��ԭ�Ӽ۵����Ų�Ϊns2npn+2��Y���ڶ�����Ԫ�أ���n=2ʱ��Y��OԪ�أ���n=3ʱY��ClԪ�أ�
Ԫ��Z��������Ԫ��Z���Ȼ�������¾�ΪҺ̬���ֱ�ΪCS2��CCl4�����ߵ���Է�������֮��Ϊ38��77����Z��CԪ�أ�X��Y�ڲ�ͬ�����¿��γ�X2Y��X2Y2���ֹ�̬�����ӦΪNa2O��Na2O2������XΪNaԪ�أ�YΪOԪ�أ�������ԭ������֮��Ϊ25����Z��ԭ������Ϊ25-11-8=6��ӦΪCԪ�أ�O��C�ڲ�ͬ�����¿��γ�CO��CO2������̬������ݴ˽��
��� �⣺Ԫ��Y��ԭ�Ӽ۵����Ų�Ϊns2npn+2��Y���ڶ�����Ԫ�أ���n=2ʱ��Y��OԪ�أ���n=3ʱY��ClԪ�أ�
Ԫ��Z��������Ԫ��Z���Ȼ�������¾�ΪҺ̬���ֱ�ΪCS2��CCl4�����ߵ���Է�������֮��Ϊ38��77����Z��CԪ�أ�X��Y�ڲ�ͬ�����¿��γ�X2Y��X2Y2���ֹ�̬�����ӦΪNa2O��Na2O2������XΪNaԪ�أ�YΪOԪ�أ�������ԭ������֮��Ϊ25����Z��ԭ������Ϊ25-11-8=6��ӦΪCԪ�أ�O��C�ڲ�ͬ�����¿��γ�CO��CO2������̬�����
��1��������������֪��XΪNa��YΪO��ZΪC���ʴ�Ϊ��Na��O��C��
��2��Na2O2Ϊ���ӻ�����������ΪNa+��O22-���������Ӽ������ۼ����ʴ�Ϊ�����ӣ�Na+��O22-�����Ӽ������ۼ���
��3��CS2��CO2�ļ۵�����Ŀ��ȣ�Ϊ�ȵ����壬�ṹ���ƣ�Ϊֱ���η��ӣ�Cԭ�Ӳ�ȡsp�ӻ���CCl4��Cԭ����4��Clԭ���γ�4�����ۼ���û�й¶Ե��ӣ�Ϊ��������ṹ��Cԭ�Ӳ�ȡsp3�ӻ���
CS2�ĽṹʽΪS=C=S������2���Ҽ���2���м���
�ʴ�Ϊ��ֱ���Σ��������壻sp��sp3��2���Ҽ���2���м���
��4��������Zԭ������Χ��4��Zԭ���γ���������ṹ��������Zԭ�ӵ���λ��Ϊ4��������Zԭ����ĿΪ4+8��$\frac{1}{8}$+6��$\frac{1}{2}$=8����������Ϊ$\frac{8��12}{{N}_{A}}$g�������ܶȦ�=$\frac{8��12}{{N}_{A}}$g�£�acm��3=$\frac{96}{{a}^{3}•{N}_{A}}$g��cm-3��
�ʴ�Ϊ��4��$\frac{96}{{a}^{3}•{N}_{A}}$g��cm-3��
���� �����Ƕ����ʽṹ�Ŀ��飬�漰Ԫ�ص��ƶϡ����ӵĹ��͡��ӻ���ʽ����ѧ������������ȣ��ƶ�Ԫ���ǽ���ؼ�����Ŀ�Ѷ��еȣ�

 ̽���빮�̺��Ͽ�ѧ����������ϵ�д�
̽���빮�̺��Ͽ�ѧ����������ϵ�д�| A�� | ��ϩ | B�� | ��Ȳ | C�� | ��Ȳ | D�� | ��Ȳ |
| A�� | CH2=CH-CH2-CH3��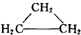 | B�� | 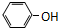 �� ��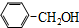 | ||
| C�� | ������ɶ�Ϊ CnH2nO2 ������ | D�� | CH3CH2Cl��CH3CH2CH2Cl |
| A�� | MnO4-������������Ӧ | |
| B�� | �������뻹ԭ�������ʵ���֮��Ϊ1��3 | |
| C�� | ����ʽ�������е�Ӧ����OH- | |
| D�� | ���������뻹ԭ�������ʵ���֮����5��2 |
| A�� | ʯ��ˮ | B�� | CO | C�� | ���� | D�� | C2H5OH |
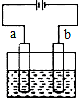
| A�� | �������Һ��Cl-��a�缫Ǩ�� | |
| B�� | ��װ���ܽ���ѧ��ת��ɵ��� | |
| C�� | b�缫�Ϸ���������Ӧ | |
| D�� | ��bΪ������b�缫�ķ�ӦʽΪ��Fe-2e-�TFe2+ |
| Ԫ�ر�� Ԫ������ | �� | �� | �� | �� | �� | �� | �� |
| ԭ�Ӱ뾶��10-10m�� | 0.74 | 1.60 | 1.52 | 1.10 | 0.99 | 1.86 | 0.75 |
| ��������ϼ� | +2 | +1 | +5 | +7 | +1 | +5 | |
| ������ϼ� | -2 | -3 | -1 | -3 |
��1��Ԫ�آ������ڱ��е�λ�õ������ڢ�A�壻
��2��Ԫ�آ���Ԫ�آ���Ƚϣ���̬�⻯����ȶ�����
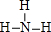 ����ṹʽ����
����ṹʽ������3��Ԫ�آ��γɵ�+3��+5�۵��Ȼ����У���ԭ�Ӿ��ﵽ8�����ȶ��ṹ�Ļ�������PCl3��д��ѧʽ����
��4������������ȷ����A��
A���⻯��ķе�Ϊ�ܣ���B��������γɵĻ������������
C����ϡ���ᷴӦ���ʢڱȵ��ʢ� D������������Ӧˮ����ļ��Ԣۣ���
 ���Ƿǽ�������ǿ��Ԫ�أ��ش��������⣺
���Ƿǽ�������ǿ��Ԫ�أ��ش��������⣺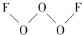 ��������ԭ�Ӳ��õĹ���ӻ���ʽ��sp3����Ԫ�صĻ��ϼ���-1��
��������ԭ�Ӳ��õĹ���ӻ���ʽ��sp3����Ԫ�صĻ��ϼ���-1��