��Ŀ����
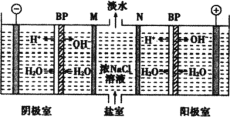
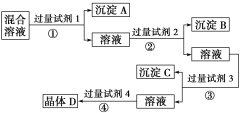
����Ŀ������NaCl��Na2SO4��NaNO3�Ļ����Һ��ѡ���ʵ����Լ�����ת��Ϊ��Ӧ�ij�������壬�Ӷ�ʵ��Cl����SO42-��NO3-�ķ��룬ʵ�������ͼ��ʾ��
��ش��������⣺
��1��д������ʵ������������Լ������ƣ�
�Լ�1__���Լ�2__���Լ�4___��
��2����������Լ�3��Ŀ����___��
��3���ڼ����Լ�4��þ���D��ʵ���������___��
��4��д�����̢ڵ����ӷ���ʽ___��
���𰸡�BaCl2��Һ[��Ba(NO3)2��Һ] AgNO3��Һ ϡ���� ��ȥ������Ba2����Ag�� ��������ȴ���ᾧ������ Ag��+Cl��=AgCl��
��������
������Һ�е������ӵ����࣬��ȥ��������������ᱵ����ȥ����������������������Լ���Ϊ�Թ���������Ҫ��ȥ�����ӡ������ӣ���̼���ƣ����˺����������ȥ̼������ӡ�
��1�����ݷ�����֪���Լ�1Ϊ���ᱵ���Ȼ���Ҳ�ɣ��Լ�2Ϊ���������Լ�4Ϊ���
��2���Լ�3Ϊ̼���ƣ��������ʱ���ɳ�ȥ��Һ���Թ�����Ba2����Ag����
��3�������Լ�4�����ҺΪ�����ƣ��ɾ�����������ȴ���ᾧ�����˿ɻ�������ƾ��壻
��4���Լ�2Ϊ����������ȥ��Һ�е������ӣ���Ӧ�����ӷ���ʽΪAg��+Cl��=AgCl����

����Ŀ��I. ��֪4��ʱ���ֻ�������ˮ�к�Һ���е��ܽ�����±���
AgNO3 | Ba(NO3)2 | AgCl | BaCl2 | |
H2O(Һ) | 170g | 9.2g | 1.5��10-4g | 33. 3g |
NH3(Һ) | 86g | 97.2g | 0.8g | 0g |
��������������ˮ���γɸ��ֽⷴӦ�����ӷ���ʽΪ_____________________����Һ�����γɸ��ֽⷴӦ�Ļ�ѧ����ʽΪ______________________________��
II. ��������ʮ�����ʣ���H2 ���� ��CaO ��CO2 ��H2SO4 ��Ba(OH)2 �� ���ɫ����������Һ�� ����ˮ ��ϡ���� ��Al2(SO4)3
��1������ʮ������������������֮��ɷ������ӷ�Ӧ��H+��OH��![]() H2O�������ӷ�Ӧ��Ӧ�Ļ�ѧ����ʽΪ _____________________________________________
H2O�������ӷ�Ӧ��Ӧ�Ļ�ѧ����ʽΪ _____________________________________________
��2������ˮ�еĵ��뷽��ʽΪ ____________________________________
��3����������ͨ��������Һ�з�Ӧ�����ӷ���ʽΪ_____________________________
��������ͨ��������Һ�з�Ӧ�����ӷ���ʽΪ_______________________________
��4�����������̼��������Һ��Ӧ���ӷ���ʽ��_________________________________
��5��������������Ӧ�Ļ�ѧ����ʽΪ��Al + 4HNO3 = Al(NO3)3 + NO�� + 2H2O��
��˫������������ת�Ƶķ������Ŀ__________________________________________��
����5.4g Al������Ӧʱ��ת�Ƶ��ӵ���ĿΪ ____________ ��