题目内容
已知下列热化学方程式:Fe2O3(s)+3CO(g)=2Fe(s)+3CO2(g)  H="-24.8" kJ·mol-1
H="-24.8" kJ·mol-1
3Fe2O3(s)+CO(g)=2Fe3O4(s)+CO2(g) H="-47.2" kJ·mol-1
H="-47.2" kJ·mol-1
Fe3O4(s)+CO(g)="3FeO" (s)+CO2(g) H="+640.5" kJ·mol-1
H="+640.5" kJ·mol-1
则14g CO气体与足量FeO充分反应得到Fe单质和CO2气体时的反应热为( )
 H="-24.8" kJ·mol-1
H="-24.8" kJ·mol-13Fe2O3(s)+CO(g)=2Fe3O4(s)+CO2(g)
 H="-47.2" kJ·mol-1
H="-47.2" kJ·mol-1Fe3O4(s)+CO(g)="3FeO" (s)+CO2(g)
 H="+640.5" kJ·mol-1
H="+640.5" kJ·mol-1则14g CO气体与足量FeO充分反应得到Fe单质和CO2气体时的反应热为( )
| A.-218 kJ·mol-1 | B.-109kJ·mol-1 | C.+218 kJ·mol-1 | D.+109 kJ·mol-1 |
B
根据①Fe2O3(s)+3CO(g)=2Fe(s)+3CO2(g) △H=-24.8 kJ/mol
②3Fe2O3(s)+ CO(g)==2Fe3O4(s)+ CO2(g) △H=-47.2 kJ/mol
③Fe3O4(s)+CO(g)==3FeO(s)+CO2(g) △H=+640.5 kJ/mol
由①×3-②-③×2可得热化学方程式为:
CO(g)+FeO(s)=" Fe(s)" + CO2(g) △H=-218.00 kJ/mol,故答案为B
②3Fe2O3(s)+ CO(g)==2Fe3O4(s)+ CO2(g) △H=-47.2 kJ/mol
③Fe3O4(s)+CO(g)==3FeO(s)+CO2(g) △H=+640.5 kJ/mol
由①×3-②-③×2可得热化学方程式为:
CO(g)+FeO(s)=" Fe(s)" + CO2(g) △H=-218.00 kJ/mol,故答案为B

练习册系列答案
相关题目
 O2(g)=H2O(1)△H = ―285.8kJ/mol
O2(g)=H2O(1)△H = ―285.8kJ/mol =2H2O(g) △H2=-483.6 kJ·
=2H2O(g) △H2=-483.6 kJ·
 的ΔH为____________________。
的ΔH为____________________。 =2H2O(g);△H2=--Q2 kJ·
=2H2O(g);△H2=--Q2 kJ·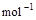 ,
, ;HCl(aq)与NaOH反应的
;HCl(aq)与NaOH反应的 ,则HCN在水溶液中电离的
,则HCN在水溶液中电离的 等于
等于
