��Ŀ����
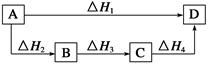
��ͬ��ͬѹ�£����и����Ȼ�ѧ����ʽ��Q2 > Q1��Q2��Q1��Ϊ��ֵ�����ǣ� ��
| A��2H2(g)+O2(g)=2H2O(l)��H= ��Q1kJ��mol-1�� 2H2(g)+O2(g)=2H2O(g)��H=��Q2kJ��mol-1 |
| B��S(g)+O2(g)=SO2(g)��H = ��Q1 kJ��mol-1�� S(s)+O2(g)=SO2(g)��H=��Q2kJ��mol-1 |
| C��C(s)+1/2O2(g)=CO(g)��H = ��Q1 kJ��mol-1�� C(s)+O2(g)=CO2(g)��H=��Q2kJ��mol-1 |
| D��H2(g)+Cl2(g)=2HCl(g)��H = ��Q1kJ��mol-1�� 1/2H2(g)+1/2Cl2(g)="HCl(g)" ��H= ��Q2kJ��mol-1 |
C
���鷴Ӧ�ȵĴ�С�Ƚϡ�����Һ̬ˮ������������̬ˮ����������������ȼ������Һ̬ˮ���ȶ࣬����ѡ��A��Q1 > Q2����̬S���������ڹ�̬S��������������̬Sȼ�շ��ȶ࣬���ѡ��B��Q1 > Q2��̼��ȫȼ�շ��ȶ࣬����ѡ��C��Q2 > Q1���μӷ�Ӧ������Խ�࣬�ų�������Խ�࣬���ѡ��D��Q1 > Q2����ѡC��

��ϰ��ϵ�д�
�����Ŀ
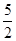 O2(g) == 2CO2(g)��H2O(l) ��H1��-1301.0 kJ?mol-1
O2(g) == 2CO2(g)��H2O(l) ��H1��-1301.0 kJ?mol-1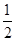 O2(g) == H2O(1) ��H3 =" -285.8" kJ��mol-1
O2(g) == H2O(1) ��H3 =" -285.8" kJ��mol-1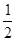 O2��g��====H2O��g�� ��H1=-Q1 kJ��mol-1
O2��g��====H2O��g�� ��H1=-Q1 kJ��mol-1 H="-24.8" kJ��mol-1
H="-24.8" kJ��mol-1