��Ŀ����
���и����Ȼ�ѧ����ʽ�У���ѧ��Ӧ�Ħ�Hǰ�ߴ��ں��ߵ���(����)
��C(s)��O2(g)===CO2(g)����H1�� C(s)��1/2O2(g)===CO(g)����H2
��NaOH(aq)��HCl(aq)===NaCl(aq)��H2O(l)����H3��
NaOH(aq)��HNO3(aq)===NaNO3(aq)��H2O(l)����H4
��H2(g)��1/2O2(g)===H2O(l)����H5��2H2(g)��O2(g)===2H2O(l)����H6
��CaCO3(s)===CaO(s)��CO2(g)����H7��CaO(s)��H2O(l)===Ca(OH)2(s)����H8
��C(s)��O2(g)===CO2(g)����H1�� C(s)��1/2O2(g)===CO(g)����H2
��NaOH(aq)��HCl(aq)===NaCl(aq)��H2O(l)����H3��
NaOH(aq)��HNO3(aq)===NaNO3(aq)��H2O(l)����H4
��H2(g)��1/2O2(g)===H2O(l)����H5��2H2(g)��O2(g)===2H2O(l)����H6
��CaCO3(s)===CaO(s)��CO2(g)����H7��CaO(s)��H2O(l)===Ca(OH)2(s)����H8
| A���� | B���ڢ� | C���ۢ� | D���٢ڢ� |
C
̼��ȫȼ�շų��������࣬������Խ�࣬��HԽС�����Ԣ��Ц�Hǰ��С�ں��ߣ����ж����к��ȣ���H��ȣ����ĵ�����Խ�࣬�ų�������Խ�࣬ͬ������Խ�࣬��HԽС�����Ԣ��Ц�Hǰ�ߴ��ں��ߣ�����ǰ�������ȷ�Ӧ�������Ƿ��ȷ�Ӧ����Hǰ�ߴ��ں��ߣ���ѡC��

��ϰ��ϵ�д�
�����Ŀ
 O2(g)=H2O(l) ��H=��285.84kJ��mol��1
O2(g)=H2O(l) ��H=��285.84kJ��mol��1  2SO3(g)��Ӧ���̵������仯��ͼ��ʾ����ش��������⣺
2SO3(g)��Ӧ���̵������仯��ͼ��ʾ����ش��������⣺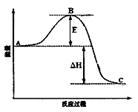
 O2(g)==H2O(l) ��H3= ��285 kJ��mol-1
O2(g)==H2O(l) ��H3= ��285 kJ��mol-1 H="-24.8" kJ��mol-1
H="-24.8" kJ��mol-1 CH3OH(g) ��H1
CH3OH(g) ��H1

