��Ŀ����
ÿ��2�֣���12�֣���ѧ��Ӧ��Ϊ�����ṩ����
������Һ��������Ҫ�ɷ�֮һ�Ƕ��飨C4H10�������³�ѹ�£���5.8 kg������ȫȼ�ղ����ɶ�����̼��Һ̬ˮʱ���ų�����Ϊ2.9��105kJ����д������ȼ�յ��Ȼ�ѧ����ʽ��____________________________________________����֪1molҺ̬ˮ����ʱ��Ҫ����44 kJ����������Ӧ��
 �Ħ�HΪ____________________��
�Ħ�HΪ____________________��
����Ǧ�����ǻ�ѧ��Դ����缫���Ϸֱ���Pb��PbO2���������ҺΪϡ���ᡣ����ʱ���õ�ص��ܷ�ӦΪPbO2+Pb+2H2SO4=2PbSO4+2H2O��������������жϣ�
��1�����صĸ�����______����缫��ӦʽΪ______________________________��
��2�����ص������缫��ӦʽΪ_________________________________��
��3�����ع���ʱ�����е������Һ��pH______�����������С�����䡱����
������Һ��������Ҫ�ɷ�֮һ�Ƕ��飨C4H10�������³�ѹ�£���5.8 kg������ȫȼ�ղ����ɶ�����̼��Һ̬ˮʱ���ų�����Ϊ2.9��105kJ����д������ȼ�յ��Ȼ�ѧ����ʽ��____________________________________________����֪1molҺ̬ˮ����ʱ��Ҫ����44 kJ����������Ӧ��
 �Ħ�HΪ____________________��
�Ħ�HΪ____________________������Ǧ�����ǻ�ѧ��Դ����缫���Ϸֱ���Pb��PbO2���������ҺΪϡ���ᡣ����ʱ���õ�ص��ܷ�ӦΪPbO2+Pb+2H2SO4=2PbSO4+2H2O��������������жϣ�
��1�����صĸ�����______����缫��ӦʽΪ______________________________��
��2�����ص������缫��ӦʽΪ_________________________________��
��3�����ع���ʱ�����е������Һ��pH______�����������С�����䡱����
��ÿ��2�֣���12�֣�
����2 C4H10(g)+13O2(g)=8CO2(g)+10H2O(l) ��H=��5800kJ��mol-1 ��2680kJ��mol-1
����1��Pb Pb��2e+-SO42-=PbSO4��
��2��PbO2+4H++SO42-+2e-=PbSO4+2H2O�� ��3������
����2 C4H10(g)+13O2(g)=8CO2(g)+10H2O(l) ��H=��5800kJ��mol-1 ��2680kJ��mol-1
����1��Pb Pb��2e+-SO42-=PbSO4��
��2��PbO2+4H++SO42-+2e-=PbSO4+2H2O�� ��3������
����5.8 kg������ȫȼ�ղ����ɶ�����̼��Һ̬ˮʱ���ų�����Ϊ2.9��105kJ������1mol������ȫȼ�շų���������2.9��105kJ��100��2.9��103kJ�����Է�Ӧ���Ȼ�ѧ����ʽ��2C4H10(g)+13O2(g)=8CO2(g)+10H2O(l) ��H=��5800kJ��mol-1��1molҺ̬ˮ����ʱ��Ҫ����44 kJ������������5molҺ̬ˮ����ʱ��Ҫ����220 kJ�����������C4H10(g)+13/2O2(g)=4CO2(g)+5H2O(l) ��H=����2900��220��kJ/mol����2680kJ/mol��
����1��ԭ����и���ʧȥ���ӣ����Ը����ܷ�Ӧʽ��֪�����صĸ�����Pb���缫��Ӧʽ��Pb��2e+-SO42-=PbSO4��
��2�������ǵõ����ӵģ����Զ�����Ǧ�������õ����ӣ��缫��Ӧʽ��PbO2+4H++SO42-+2e-=PbSO4+2H2O��
��3�������ܷ�Ӧʽ��֪�����ع���ʱ������ϡ���ᣬ������Һ��pH����
����1��ԭ����и���ʧȥ���ӣ����Ը����ܷ�Ӧʽ��֪�����صĸ�����Pb���缫��Ӧʽ��Pb��2e+-SO42-=PbSO4��
��2�������ǵõ����ӵģ����Զ�����Ǧ�������õ����ӣ��缫��Ӧʽ��PbO2+4H++SO42-+2e-=PbSO4+2H2O��
��3�������ܷ�Ӧʽ��֪�����ع���ʱ������ϡ���ᣬ������Һ��pH����

��ϰ��ϵ�д�
 ��������ϵ�д�
��������ϵ�д� ����˼ά����ѵ����ʱ��ѧ��ϵ�д�
����˼ά����ѵ����ʱ��ѧ��ϵ�д�
�����Ŀ
 O2(g)
O2(g) 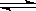 SO3(g) ��H =" �D98.32" kJ��mol��1���������г���2molSO2 ��1molO2��ַ�Ӧ�����շų�������Ϊ
SO3(g) ��H =" �D98.32" kJ��mol��1���������г���2molSO2 ��1molO2��ַ�Ӧ�����շų�������Ϊ 2SO3(g)��Ӧ���̵������仯��ͼ��ʾ����ش��������⣺
2SO3(g)��Ӧ���̵������仯��ͼ��ʾ����ش��������⣺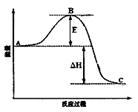
 O2(g)==H2O(l) ��H3= ��285 kJ��mol-1
O2(g)==H2O(l) ��H3= ��285 kJ��mol-1 H="-24.8" kJ��mol-1
H="-24.8" kJ��mol-1