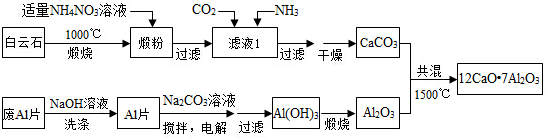
��Ŀ����
16����ͼ1����ѧ��ѧ�г��õ�ʵ��װ�ã�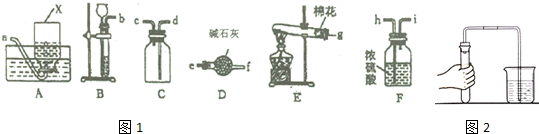
��1��װ��A�������ռ�O2��H2���壨�����ֳ�������Ļ�ѧʽ����
��2��ʵ������װ��B��ȡO2�Ļ�ѧ����ʽ��2H2O2$\frac{\underline{\;MnO_2\;}}{\;}$2H2O+O2����
��3������װ��B��ȡH2����F�������ɵ�H2����Cװ���ռ������H2����������������ҵ�����д����Ӧװ�ýӿڵ�����˳��b��h��i��c��d��
��4����ͼ2��ʵ���Ҽ�������װ��������ʾ��ͼ����������������װ�����������õIJ���������
�������������Թ�
���ܿ��������ݳ����ɿ��ֵ�������һ��ˮ��������������һ��ʱ�䲻�½�
��5������ɫ��ѧ��ʵ�鰲ȫ�ĽǶȿ��ǣ�����CO��ԭCuOʵ���β�������þƾ��Ƶ�ȼβ�������������ռ�β������������
���� ��1��װ��A���ռ�������ˮ�����壻
��2����װ��B��ȡO2��Ϊ������Һ�岻����װ�ã���ѡ�����������������⣻
��3����װ��B��ȡH2����F�������ɵ�H2����Cװ���ռ������H2�������ʱ���ܳ����̳����������������ſ������ռ���
��4����������װ�������ԣ��ɼ��ȹ۲�ˮ�����ݣ�
��5��CO�ж�����ȼ���������Ķ�����̼����ֹCO��Ⱦ������
��� �⣺��1��װ��A���ռ�������ˮ�����壬��O2��H2���ʴ�Ϊ��O2��H2��
��2����װ��B��ȡO2��Ϊ������Һ�岻����װ�ã���ѡ�����������������⣬��ӦΪ2H2O2$\frac{\underline{\;MnO_2\;}}{\;}$2H2O+O2�����ʴ�Ϊ��2H2O2$\frac{\underline{\;MnO_2\;}}{\;}$2H2O+O2����
��3����װ��B��ȡH2����F�������ɵ�H2����Cװ���ռ������H2�������ʱ���ܳ����̳����������������ſ������ռ�����������������ҵ�������Ӧװ�ýӿڵ�����˳��Ϊb��h��i��c��d���ʴ�Ϊ��h��i��c��d��
��4����������װ�������ԣ��ɼ��ȹ۲�ˮ�����ݣ�����Ϊ���������Թܣ�����Ϊ���ܿ��������ݳ����ɿ��ֵ�������һ��ˮ��������������һ��ʱ�䲻�½���
�ʴ�Ϊ�����������Թܣ����ܿ��������ݳ����ɿ��ֵ�������һ��ˮ��������������һ��ʱ�䲻�½���
��5��CO�ж�����ȼ���������Ķ�����̼����ֹCO��Ⱦ��������β����������Ϊ�þƾ��Ƶ�ȼβ�������������ռ�β�������ʴ�Ϊ���þƾ��Ƶ�ȼβ�������������ռ�β������
���� ���⿼��ʵ��װ�õ��ۺ�Ӧ�ã�Ϊ��Ƶ���㣬������ѧ���ķ�����ʵ�������Ŀ��飬ע�ⳣ����������ʼ��Ʊ�ԭ����ʵ��װ�õ����õȣ���Ŀ�ѶȲ���

 53���ò�ϵ�д�
53���ò�ϵ�д�| A�� | NaNO2�ǻ�ԭ�� | |
| B�� | NH4Cl��NԪ�ر���ԭ | |
| C�� | ����1molN2ʱת��6mol���� | |
| D�� | �������ͻ�ԭ�������ʵ���֮����1��1 |
����һ��Fe$\stackrel{ϡ����}{��}$H2 $\stackrel{����ͭ}{��}$Cu
��������CuO$\stackrel{ϡ����}{��}$CuSO 4$\stackrel{��}{��}$Cu
���ݹ淶��ʵ�鷽���Ͳ������������ַ����Ƶõ���ͭ��������ϵΪ��������
| A�� | ��� | B�� | ����һ�� | C�� | �������� | D�� | ���ж� |
 ���и��������У�����֮��ͨ��һ����Ӧ��ʵ��ͼʾ�仯���ǣ�������
���и��������У�����֮��ͨ��һ����Ӧ��ʵ��ͼʾ�仯���ǣ�������| �������� | a | b | c | d |
| �� | CO2 | CO | C | CaCO3 |
| �� | Na2CO3 | NaOH | Na2O2 | NaHCO3 |
| �� | FeCl3 | FeCl2 | Fe | CuCl2 |
| �� | Al2O3 | NaAlO2 | Al | Al��OH��3 |
| A�� | �٢ڢ� | B�� | �ڢۢ� | C�� | �ڢ� | D�� | �ۢ� |
�ٵ�������Һ�м�������狀��γɳ���
�����������������еμ��������������
�۶�����������ѹ��������ɫҺ�壻
����ˮ�����ѻ�����������ɫ��
������ͭ��ĩ�μ�����ˮ������
����̿��ȥˮ�е���ɫ����ζ��
| A�� | �٢ڢ� | B�� | �ڢܢ� | C�� | �ܢݢ� | D�� | �٢ڢ� |
| A�� | 1 mol | B�� | 0.5 mol | C�� | 2 mol | D�� | ������ |