��Ŀ����
����Ŀ�����ڱ�ǰ�����ڵ�Ԫ��![]() ��
��![]() ��
��![]() ��
��![]() ��ԭ��������������Xԭ�ӻ�̬ʱ
��ԭ��������������Xԭ�ӻ�̬ʱ![]() ����
����![]() �����������s�����������ͬ��
�����������s�����������ͬ��![]() ԭ�ӻ�̬ʱ2pԭ�ӹ������3��δ�ɶԵĵ��ӣ�Z�ж������������һ�ֺ���ɫ���������Ϳ�ϣ�
ԭ�ӻ�̬ʱ2pԭ�ӹ������3��δ�ɶԵĵ��ӣ�Z�ж������������һ�ֺ���ɫ���������Ϳ�ϣ�![]() λ�ڵ������ڣ���ԭ�������ֻ��1�����ӣ����ڲ㶼����ȫ����״̬���ش��������⣺
λ�ڵ������ڣ���ԭ�������ֻ��1�����ӣ����ڲ㶼����ȫ����״̬���ش��������⣺
(1)Xλ�����ڱ��ĵ�_______���ڣ���______�塣
(2)Ԫ�صĵ�һ�����ܣ�X______Y(�������������ͬ)��ԭ�Ӱ뾶��X______Y��
(3)![]() ������������Ӧˮ������������ӵĿռ乹����_______(����������)��
������������Ӧˮ������������ӵĿռ乹����_______(����������)��
(4)![]() ��̬��������Ų�ʽΪ_________�������軯����Һ����
��̬��������Ų�ʽΪ_________�������軯����Һ����![]() �����ӷ���ʽΪ___________��
�����ӷ���ʽΪ___________��
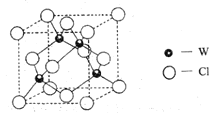
(5)Ԫ��W��һ���Ȼ��ᄃ��ľ����ṹ��ͼ��ʾ�����Ȼ���Ļ�ѧʽ��_______��������Ũ���ᷢ����������ԭ��Ӧ�����������![]() ����Ӧ�Ļ�ѧ����ʽ��_________��
����Ӧ�Ļ�ѧ����ʽ��_________��
���𰸡��� IVA �� �� ƽ�������� ![]() ��
��![]()
![]()
![]()
![]()
��������
ǰ������Ԫ��![]() ��
��![]() ��
��![]() ��
��![]() ��ԭ��������������Xԭ�ӻ�̬ʱ
��ԭ��������������Xԭ�ӻ�̬ʱ![]() ����
����![]() �����������s�����������ͬ���������Ų�ʽΪ��1s22s22p2����XΪCԪ�أ�
�����������s�����������ͬ���������Ų�ʽΪ��1s22s22p2����XΪCԪ�أ�![]() ԭ�ӻ�̬ʱ2pԭ�ӹ������3��δ�ɶԵĵ��ӣ��������Ų�ʽΪ��1s22s22p3����YΪNԪ�أ�Z�ж������������һ�ֺ���ɫ���������Ϳ�ϣ���ZΪFeԪ�أ�
ԭ�ӻ�̬ʱ2pԭ�ӹ������3��δ�ɶԵĵ��ӣ��������Ų�ʽΪ��1s22s22p3����YΪNԪ�أ�Z�ж������������һ�ֺ���ɫ���������Ϳ�ϣ���ZΪFeԪ�أ�![]() λ�ڵ������ڣ���ԭ�������ֻ��1�����ӣ����ڲ㶼����ȫ����״̬���������Ų�ʽΪ��1s22s22p63s23p63d104s1����WΪCuԪ�ء��ݴ˽��
λ�ڵ������ڣ���ԭ�������ֻ��1�����ӣ����ڲ㶼����ȫ����״̬���������Ų�ʽΪ��1s22s22p63s23p63d104s1����WΪCuԪ�ء��ݴ˽��
��1��XΪCԪ�أ�λ�����ڱ��ĵڶ����ڣ���IVA�壬�ʴ�Ϊ������IVA��
��2��XΪCԪ�أ�YΪNԪ�أ�NԪ�صĻ�̬ԭ�ӵĵ����Ų�ʽΪ��1s22s22p3��p������ڰ����״̬�����ȶ�����һ�����ܣ�C��N��ͬ���ڣ������ң�ԭ�Ӱ뾶��С��ԭ�Ӱ뾶��C��N���ʴ�Ϊ����������
��3��YΪNԪ�أ�������������Ӧˮ����ΪHNO3���������ΪNO3-������ԭ��Nԭ�ӵŶԵ�����Ϊ��![]() ���۵��Ӷ���Ϊ=
���۵��Ӷ���Ϊ=![]() ��+�¶Ե�����=0+3=3����ռ乹����ƽ�������Σ��ʴ�Ϊ��ƽ�������Ρ�
��+�¶Ե�����=0+3=3����ռ乹����ƽ�������Σ��ʴ�Ϊ��ƽ�������Ρ�
��4��ZΪFeԪ�أ�Fe3+��̬��������Ų�ʽΪ![]() ��
��![]() �������軯����Һ����Fe2+�����ӷ���ʽΪ��
�������軯����Һ����Fe2+�����ӷ���ʽΪ��![]() �����ʴ�Ϊ��
�����ʴ�Ϊ��![]() ��
��![]() ����
����
��5��WΪCuԪ�أ���ͼ��֪�������У�CuԪ�ص�ԭ�Ӹ���Ϊ4��ClԪ�ص�ԭ�Ӹ���Ϊ��![]() ������Ȼ���Ļ�ѧʽ��
������Ȼ���Ļ�ѧʽ��![]() ��
��![]() ��Ũ���ᷢ����������ԭ��Ӧ�����������
��Ũ���ᷢ����������ԭ��Ӧ�����������![]() ���䷴Ӧ�Ļ�ѧ����ʽΪ��
���䷴Ӧ�Ļ�ѧ����ʽΪ��![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��![]() ��
��

 ÿ��10���ӿ�����������������ϵ�д�
ÿ��10���ӿ�����������������ϵ�д�