��Ŀ����
����Ŀ����ͼ��ʾ��Ԫ�����ڱ�������ͼ������Ԫ�������������ڱ��е�λ�ã�����ĿҪ��ش����⣺
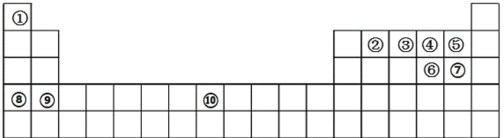
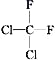
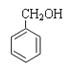
(1)Ԫ�����������γ� 16 ���ӵķ��ӣ��÷��ӵĵ���ʽΪ________________�� Ԫ�����������γ� 10 ���ӵķ��ӣ����õ���ʽ��ʾ�����ʵ��γɹ���_______________________��
(2)Ԫ�������������γɵļ��⻯������ȶ��Դ�С����˳��Ϊ___________________(�û�ѧʽ��ʾ)��
(3)��������Ԫ�������ʼ��뵽Ԫ�������������ˮ�����ϡ��Һ�У�������Ӧ�����ӷ�Ӧ����ʽΪ_______��
(4)ǦԪ����Ԫ�����ڱ��е�λ��Ϊ________________��Ǧ�������ǵ��͵Ķ��ε�أ����������й㷺��Ӧ�ã���ŵ�ʱ�����ĵ缫��ӦʽΪ________________���ŵ�һ��ʱ��������������� 6.4g�������ʱ�䣬ת�Ƶ������ʵ���Ϊ__________________mol��
���𰸡�![]()
![]() H2S��H2O��HF 3Fe��2
H2S��H2O��HF 3Fe��2![]() ��8H+= 2Fe2+��2NO����4H2O �������ڵ�IVA�� PbO2��2e����4H+��
��8H+= 2Fe2+��2NO����4H2O �������ڵ�IVA�� PbO2��2e����4H+��![]() =PbSO4��2H2O 0.2
=PbSO4��2H2O 0.2
��������
�١���Ԫ�طֱ���H��C��N��O��F��S��Cl��K��Ca��Fe���ǽ���Խǿ�����⻯����ȶ���Խǿ��
(1)Ԫ�����������γ�16���ӵķ��ӣ��÷���ΪC2H4��,�����ʽΪ![]() ��Ԫ�����������γ�10���ӵķ���ΪNH3���õ���ʽ��ʾ�����ʵ��γɹ���
��Ԫ�����������γ�10���ӵķ���ΪNH3���õ���ʽ��ʾ�����ʵ��γɹ���![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��![]() ��
��
(2)Ԫ�������������γɵļ��⻯��ΪH2O��HF��H2S�����ݷǽ���Խǿ�����⻯���ȶ���Խǿ��������ߵ����ȶ��Դ�С����˳��ΪH2S��H2O��HF���ʴ�Ϊ��H2S��H2O��HF��
(3)��������Ԫ�������ʼ��뵽Ԫ�������������ˮ�����ϡ��Һ�У������������뵽ϡ�����з�Ӧ��������������һ��������ˮ��������Ӧ�����ӷ�Ӧ����ʽΪ3Fe��2![]() ��8H+= 2Fe2+��2NO����4H2O���ʴ�Ϊ��3Fe��2
��8H+= 2Fe2+��2NO����4H2O���ʴ�Ϊ��3Fe��2![]() ��8H+= 2Fe2+��2NO����4H2O��
��8H+= 2Fe2+��2NO����4H2O��
(4)ǦԪ����̼Ԫ��ͬ�壬���Ǧ��Ԫ�����ڱ��е�λ��Ϊ�������ڵ�IVA�壬Ǧ�������ǵ��͵Ķ��ε�أ����������й㷺��Ӧ�ã���ŵ�ʱ������PbO2�õ����Ӻ������ӡ���������ӷ�Ӧ��������Ǧ��ˮ����缫��ӦʽΪPbO2��2e����4H+��![]() =PbSO4��2H2O���ŵ�һ��ʱ���������PbO2��ΪPbSO4��1mol PbO2��Ӧ����1mol PbSO4����������64g��ת��2mol���ӣ������������� 6.4g�������ʱ�䣬ת�Ƶ������ʵ���Ϊ
=PbSO4��2H2O���ŵ�һ��ʱ���������PbO2��ΪPbSO4��1mol PbO2��Ӧ����1mol PbSO4����������64g��ת��2mol���ӣ������������� 6.4g�������ʱ�䣬ת�Ƶ������ʵ���Ϊ![]() ���ʴ�Ϊ���������ڵ�IVA�壻PbO2��2e����4H+��
���ʴ�Ϊ���������ڵ�IVA�壻PbO2��2e����4H+��![]() =PbSO4��2H2O��0.2��
=PbSO4��2H2O��0.2��

����Ŀ��Ϊ��̽����ѧ��Ӧ���ʺͻ�ѧ��Ӧ�ȵ��й�����,ij�о�С�����������ʵ��:
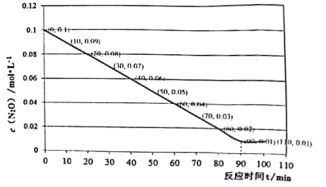
ʵ��һ:Ϊ̽����Ӧ��Ũ�ȶԻ�ѧ��Ӧ���ʵ�Ӱ��,��Ƶ�ʵ�鷽�����±���
(��֪I2+2S2O![]() ==S4O
==S4O![]() +2I- ,����Na2S2O3��Һ������,��S2O
+2I- ,����Na2S2O3��Һ������,��S2O![]() ��S4O
��S4O![]() ��Ϊ��ɫ)
��Ϊ��ɫ)
ʵ����� | ���V/mL | ��ɫ��ȥʱ��/s | |||
Na2S2O3��Һ | ������Һ | ��ˮ | ˮ | ||
�� | 10.0 | 2.0 | 4.0 | 0.0 | t1 |
�� | 8.0 | 2.0 | 4.0 | 2.0 | t2 |
�� | 6.0 | 2.0 | 4.0 | Vx | t3 |
(1)����Vx=_________mL,t1��t2��t3�Ĵ�С��ϵ��___________________
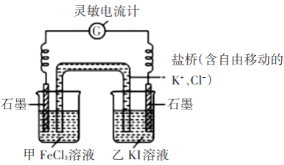
ʵ���:ȡ5 mL 0.1 mol��L-1��KI��Һ���Թ���,�μ�0.1 mol��L-1 FeCl3��Һ2 mL,�������·�Ӧ:2Fe3++2I-=2Fe2+ +I2,Ϊ֤���÷�Ӧ����һ������,�������������ʵ��:
��ȡ������ӦҺ,�μ�AgNO3��Һ,������������ɫ����(AgI)��
����ȡ������ӦҺ,�μ�����CCl4 ,��,����CCl4����dz��ɫ��
���ݢ٢ڵ�����,/span>���ǵó�����:�÷�Ӧ����һ���Ŀ�����,��һ�������»�ﵽ��Ӧ�ȡ�
(2)ָ����ʦָ������ʵ��ٲ�����,��ԭ����_______________ ; ��ĸĽ�������________(��Ҫд���������Լ�������)��
(3)ʵ����ʺϼ�������I2�϶�����������һ�ּ��ķ������������ؼ����Ƿ�������I2,���ַ������õ��Լ���_______________________��