��Ŀ����
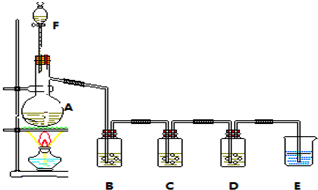
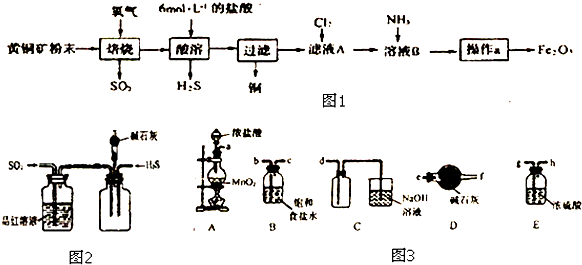
17����ͭ�����Ҫ�ɷ��� CuFeS2����Ԫ����-2�ۣ���Ԫ����+2�ۣ���ʵ�������û�ͭ��Ϊԭ����ȡ����Cu��Fe2O3��������ͼ1��ʾ��
��1��������������д�����ջ�ͭ��Ļ�ѧ����ʽCuFeS2+O2$\frac{\underline{\;����\;}}{\;}$Cu+FeS+SO2
��2������Ӧ�����в�����SO2 ��H2S ͨ����ͼ2��ʾװ���м������ǵ����ʣ���ʵ��֤��SO2 ����Ư�� �Ժ������ԣ�
��3����ѡ����ͼ3��ʾ�IJ���װ����ʵ��������MnO2 ��Ũ����Ϊԭ����ȡ�����������������
�ٰ��������������ң����ܿ�����˳��Ϊa��b��c��g��h��d��
��װ�����Ӻú���װҩƷǰ�������װ�õ������ԣ����巽���ǹرշ�Һ©���Ļ������������ܲ���ˮ�У���Բ����ƿ����������ĩ�˲������ݣ�ֹͣ���ȣ�����������һ��ˮ���γɣ�˵��װ�ò�©����
��4��Fe2O3 ���������죬�����Ƶõ� Fe2O3����;Ϊ��ɫ�����Ϳ����ʹ��l0g��ͭ���ĩ���Ƶ�12g
Fe2O3����û�ͭ���к� CuFeS2������������91.2%�����������Ӧ����ȫ�����ҹ���������������ģ���
���� ��1�����ջ�ͭ��Ӧ������������ͭ�Ͷ�������
��2��SO2ͨ��Ʒ����Һ��֤��SO2����Ư���ԣ�SO2ͨ��H2S��Һ������S����ɫ������֤��SO2���������ԣ�
��3��MnO2��Ũ���ᷴӦ����Cl2��MnCl2��H2O�����ӷ���ʽΪ��MnO2+4H++2Cl?$\frac{\underline{\;\;��\;\;}}{\;}$Mn2++Cl2��2H2O��
�����ɵ�Cl2����HCl��H2O������a����b��c��ͨ������ʳ��ˮ��ȥHCl����������g��h��ͨ��Ũ���ᣬ��ȥH2O��������d�������ռ������������������
�����ü����������͵�ԭ������װ�������Եļ��飬���Լ��鷽��Ϊ���رշ�Һ©���Ļ����������ܲ���ˮ�У���Բ����ƿ��������ĩ�˲������ݣ�ֹͣ���ȣ���������һ��ˮ���γɣ�˵��װ�ò�©����
��4��������Ϊ��������Ʊ����ᣬ����FeԪ���غ�ɵã�2CuFeS2��Fe2O3���Դ˼���û�ͭ���к�CuFeS2������������
��� �⣺��1�����ջ�ͭ��Ӧ������������ͭ�Ͷ�������Ӧ�Ļ�ѧ����ʽΪ��CuFeS2+O2$\frac{\underline{\;����\;}}{\;}$Cu+FeS+SO2 ��
�ʴ�Ϊ��CuFeS2+O2$\frac{\underline{\;����\;}}{\;}$Cu+FeS+SO2 ��
��2��SO2ͨ��Ʒ����Һ��֤��SO2����Ư���ԣ�SO2ͨ��H2S��Һ������S����ɫ������֤��SO2���������ԣ��ʴ�Ϊ��Ư�ף�������
��3��MnO2��Ũ���ᷴӦ����Cl2��MnCl2��H2O�����ӷ���ʽΪ��MnO2+4H++2Cl?$\frac{\underline{\;\;��\;\;}}{\;}$Mn2++Cl2��2H2O��
�ʴ�Ϊ��MnO2+4H++2Cl?$\frac{\underline{\;\;��\;\;}}{\;}$Mn2++Cl2��2H2O��
�����ɵ�Cl2����HCl��H2O������a����b��c��ͨ������ʳ��ˮ��ȥHCl����������g��h��ͨ��Ũ���ᣬ��ȥH2O��������d�������ռ������������������
�ʴ�Ϊ��b��c��g��h��d��
�����ü����������͵�ԭ������װ�������Եļ��飬���Լ��鷽��Ϊ���رշ�Һ©���Ļ����������ܲ���ˮ�У���Բ����ƿ��������ĩ�˲������ݣ�ֹͣ���ȣ���������һ��ˮ���γɣ�˵��װ�ò�©����
�ʴ�Ϊ���رշ�Һ©���Ļ����������ܲ���ˮ�У���Բ����ƿ��������ĩ�˲������ݣ�ֹͣ���ȣ���������һ��ˮ���γɣ�˵��װ�ò�©����
��4���������׳�Ϊ���죬����������ɫ���ᣬͿ�ϵȣ�����FeԪ���غ�ɵã�2CuFeS2��Fe2O3�����Ըû�ͭ���к�CuFeS2����������=$\frac{\frac{12g}{160g/mol}��368g/mol}{10g}$��100%=91.2%���ʴ�Ϊ��91.2%��
���� ���⿼���������Ʊ����̷����жϣ��������ʵ�����Ӧ�ã���ѧ����ʽ�����ӷ���ʽ��д���������ջ����ǹؼ�����Ŀ�ѶȽϴ�

���к͵ζ��ķ����ⶨNaOH��Na2CO3�Ļ����Һ��NaOH�ĺ� ���������ڻ��Һ�м��������BaCl2��Һ��ʹNa2CO3��ȫת���BaCO3������Ȼ���ñ�����ζ�(��֪�������ָʾ����ɫ��pH��Χ���ټ���3.1��4.4 �ڼ���4.4��6.2 �۷�̪8.2��10)��
���������ڻ��Һ�м��������BaCl2��Һ��ʹNa2CO3��ȫת���BaCO3������Ȼ���ñ�����ζ�(��֪�������ָʾ����ɫ��pH��Χ���ټ���3.1��4.4 �ڼ���4.4��6.2 �۷�̪8.2��10)��
(1)�ζ�ʱӦѡ��  ��ָʾ����
��ָʾ����
(2)�жϵ���ζ��յ��ʵ��������  ��
��
(3)���в����ᵼ���ռ���Ʒ��NaOH�����ⶨֵƫ�ߵ���
A����ƿ������ˮϴ��δ�ô���Һ��ϴ
B����ʽ�ζ���������ˮϴ��δ�ñ�Һ��ϴ
C���ڵ� ��ǰ�����ݣ��ζ���������ʧ
��ǰ�����ݣ��ζ���������ʧ
D���ζ�ǰƽ�Ӷ������ζ��������Ӷ���
(4)Ϊ�ⶨij�ռ���Ʒ��NaOH�ĺ���(����Ʒ������ΪNa2CO3)��ijͬѧ��������ʵ�飺ȷ��ȡ5.0g��Ʒ���Ƴ�250mL��Һ��Ȼ������θ�ȡ���ƺõ��ռ���Һ20.00mL������������ˮϴ������ƿ�У��ֱ�� �������BaCl2��Һ��������ƿ�и�����1��2��ָʾ������Ũ��Ϊ0.2000mol��
�������BaCl2��Һ��������ƿ�и�����1��2��ָʾ������Ũ��Ϊ0.2000mol�� L-1�������Һ���еζ���������ݼ�¼���£�
L-1�������Һ���еζ���������ݼ�¼���£�
ʵ���� | V(�ռ���Һ)/mL | V(HCl)/mL | |
������ | ĩ���� | ||
1 | 20.00 | 0.80 | 21.00 |
2 | 20.00 | 1.00 | 20.80 |
3 | 20.00 | 0.20 | 22.80 |
���ݱ������ݣ�������ռ���Ʒ�к�NaOH����������Ϊ ��(С���������λ����)

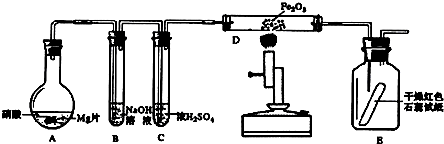
��֪�����������↑ʼ��������ȫ������pH���±���ʾ
| Fe2+ | Fe3+ | Al3+ | Mg2+ | |
| ��ʼ����ʱ��pH | 7.5 | 2.8 | 4.2 | 9.6 |
| ������ȫʱ��pH | 9.0 | 4.0 | 5 | 11 |
��1����2mol/L H2SO4��Һ�ܽ����������Ʒ����������ˮֱ���ܽ��ԭ���Ƿ�ֹFe2+ˮ�������70�����ң�������KMnO4��Һ�ζ����յ㣮������Ӧ�����ӷ���ʽΪ��2MnO4-+5H2C2O4+6H+=2Mn2++10CO2��+8H2O��MnO4-+5Fe2++8H+=5Fe3++Mn2++4H2O
��2�����·���30min��Ŀ����ʹ��Ӧ����ȫ
��3��֤����Ӧ����ȫ��ʵ�����������Ϊ��Һ���Ϻ�ɫ��ȡ�����Ϻ�ɫ��Һ���Թ��У��μ�KSCN��Һ����Һ��ɫ�����Ա仯
��4����֪������Fe��OH��3��Ksp=1.1��10-36����Һ��c��Fe3+��=1.1��10-6mol•L-1��
��5�����ճ�ֵı�־�������������ա���ȴ�������������������0.1g
��6����Ʒ��FeC2O4�ĺ���Ϊ72%��
| A�� | ��ϩ��������������е�����ԭ�Ӷ���ͬһƽ���� | |
| B�� | �����ʡ����ۡ���ά�غ���֬�����ڸ߷��ӻ����һ�������¶���ˮ�� | |
| C�� | �����������Ӧ����һ�ȼ��飬�뱽�����ᷴӦ�����������ķ�Ӧ������ͬ | |
| D�� | �ױ��ȿ�ʹ������Ȼ�̼��Һ��ɫ��Ҳ��ʹ���Ը��������Һ��ɫ |
| A�� | �����ͬ | B�� | ������� | C�� | ��������� | D�� | ԭ������� |